- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Experimental septoplasty and sensory deprivation in rat olfactory analyzer was conducted to study changes in the frequency domain of heart rate variability. Bulbectomy provokes more pronounced changes in heart rate variability in rats, compared with septoplasty simulation. After bulbectomy, there is an increase in the high frequencies, low frequencies, very low frequencies, and the LF/HF ratio.
Keywords: septoplasty, stress, bulbectomy, heart rate variability.
The olfactory analyzer plays an important role in the normal life, not only in humans, but also in animals. Disruption of its function could provoke development of a number of stress reactions. [1, 2].
Impaired olfactory function can be affected by diseases of the nasal cavity and paranasal sinuses. One of the most common conditions is the deviation of the nasal septum (DNS) [3]. Also, dysfunction of the peripheral part of the olfactory analyzer is noted in the early postoperative period in patients after septoplasty [4], which is performed in patients with DNS [5, 6].
It is believed that heart rate variability reflects the affective physiological cognitive and behavioral aspects of the pain syndrome [7, 8], as well as a number of infectious diseases [9–11], that is, the presence of a particular intensity of the inflammatory process in the body. According to modern data, the central autonomic neural network is a functional integrated model involved in tonic, reflex and adaptive control of autonomic functions [12]. Obviously, HRV can be one of the objective methods for monitoring the response of the body's neurohumoral regulatory system to various stress factors that impair the function of the olfactory analyzer [13].
The purpose of the study: to compare the degree of stressfulness of bulbectomy in rats, due to a model of sensory deprivation of the olfactory analyzer, with the simulation of septoplasty based on the analysis of heart rate variability.
The work was carried out on 20 mature male Wistar rats weighing 205.25±10.15 g. Three days before surgery, in all animals 3 metal half-rings with rounded tips were installed for subsequent fixation of the electrodes under local anesthesia with 2% lidocaine solution and general anesthesia with Zoletil 50 solution (tiletamine hydrochloride and zolazepam hydrochloride) (3 mg per 100 g of rat body weight). Three days later, an electrocardiogram (ECG) was recorded, and then surgical interventions were performed on the same day. To assess the state of the ANS, a spectral analysis of heart rate variability (HRV) was performed in rats before surgery, on days 1–6 after septoplasty modeling, and in the bulbectomy group, the indicated period corresponded to days 15–19 after surgery. The influence of humoral and supra-segmental levels of HRV regulation was assessed using the analysis of very low frequency (VLF), and the state of the PNS and SNS - using the high-frequency component of the heart rate (HF) and the low-frequency component of the heart rate (LF), respectively, as a percentage of each frequency indicator from their sums were also evaluated for the LF/HF ratio (vagosympathetic index).
Septoplasty. Zoletil 50 solution was intraperitoneally injected 10 minutes before surgery for general anesthesia at a dosage of 15 mg/kg. Modeling of septoplasty (Group 1, n=10) was performed by the standard method by zigzag scarification of the nasal mucosa (Fig. 1a) [1, 2, 7].
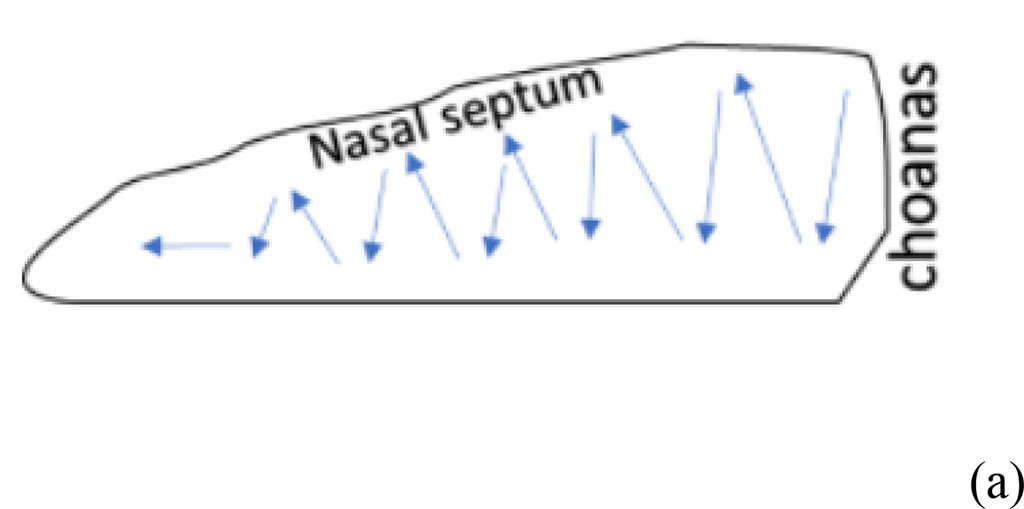
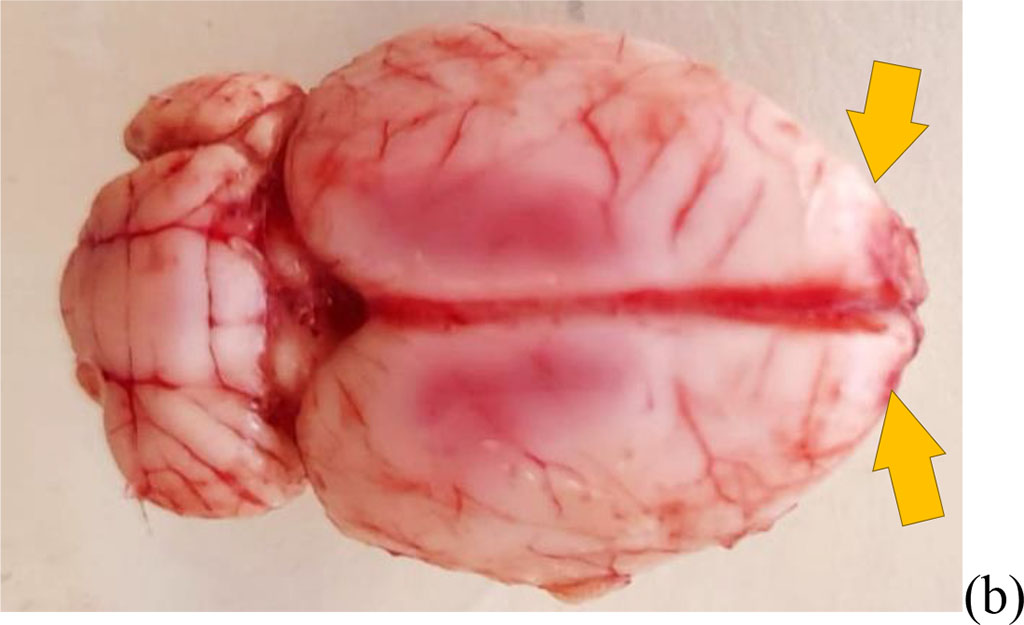
Figure 1. The result of bulbectomy in rats (yellow arrows indicate the sites of bulbectomy) (a) scheme of septoplasty simulation (b). The blue arrows indicate the direction of scarification of the nasal septum mucosa.
Bulbectomy. Bilateral bulbectomy (Fig. 1b) was performed (group 2, n=10) according to the protocol [14]. After the administration of Zoletil 100 solution at the above dosage, the head was shaved and over the olfactory bulbs in the skull, holes of 2 mm in size were made with a microborer for a needle anterior to the bregma on both sides at a distance of 2 mm from the midline of the frontal bone lying above the olfactory bulbs. Animals were excluded from the study if the bulbs were not completely removed or the frontal cortex was damaged [14]. It was important not to damage the frontal cortex. A hemostatic sponge was used to prevent bleeding from the burr holes. The antibiotic ceftriaxone powder was applied to the wound until they were closed with 7.5 mm surgical clamps. To assess the differences in the results before and after the operation, the Wilcoxon test was used, as well as between the groups, the Mann-Whitney test. Animal studies were carried out in accordance with the requirements of the "Regulations for Conducting Work Using Experimental Animals" (1984) and the " European Convention for the Protection of Vertebrate Animals used for Experimental or other Scientific Purposes" (1986). The studies were approved by the ethics committee of the RUDN MU on September 18, 2020 (protocol №1)
The bulbectomy group had significantly higher LF values throughout the follow-up period compared to the septoplasty group (p<0.001) (Fig. 2a).
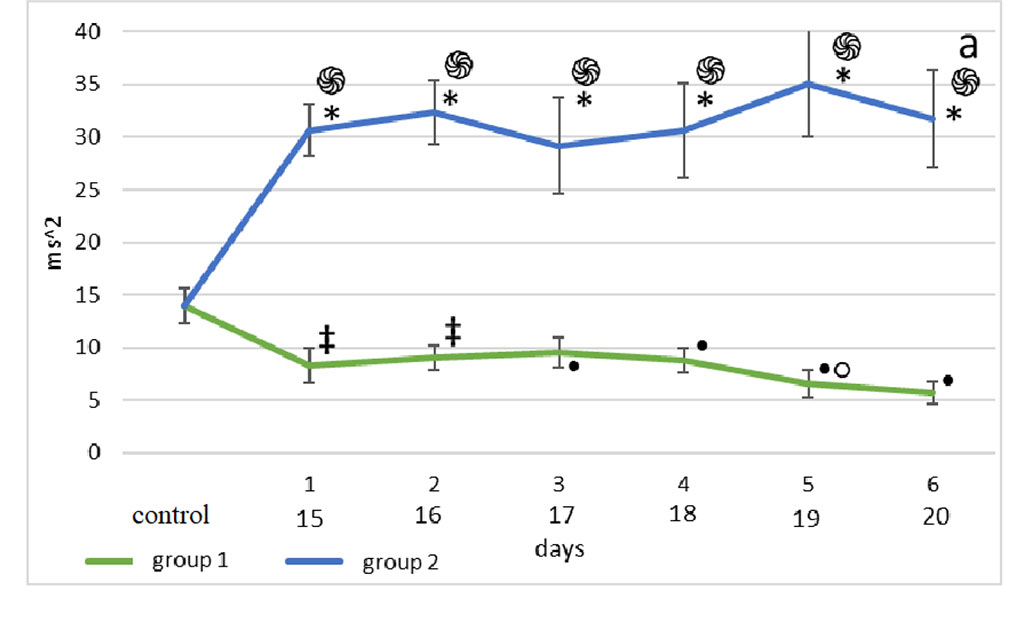
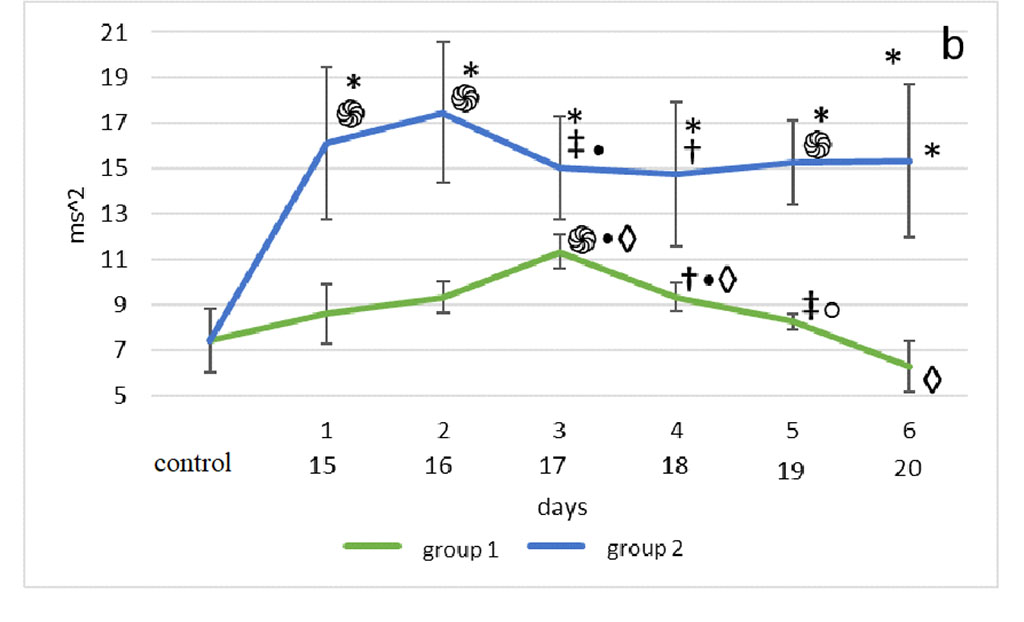
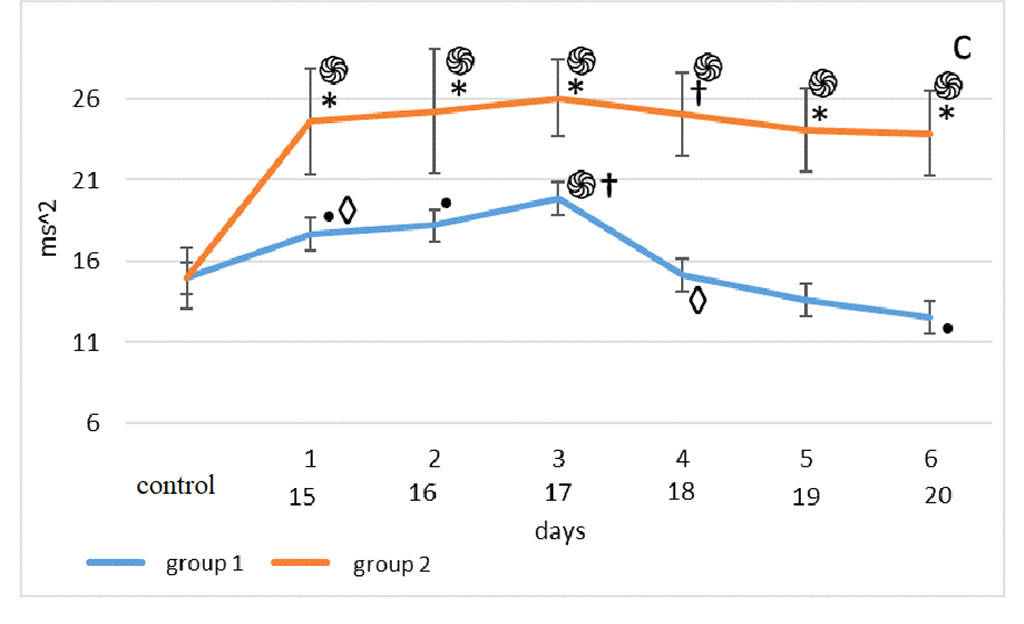
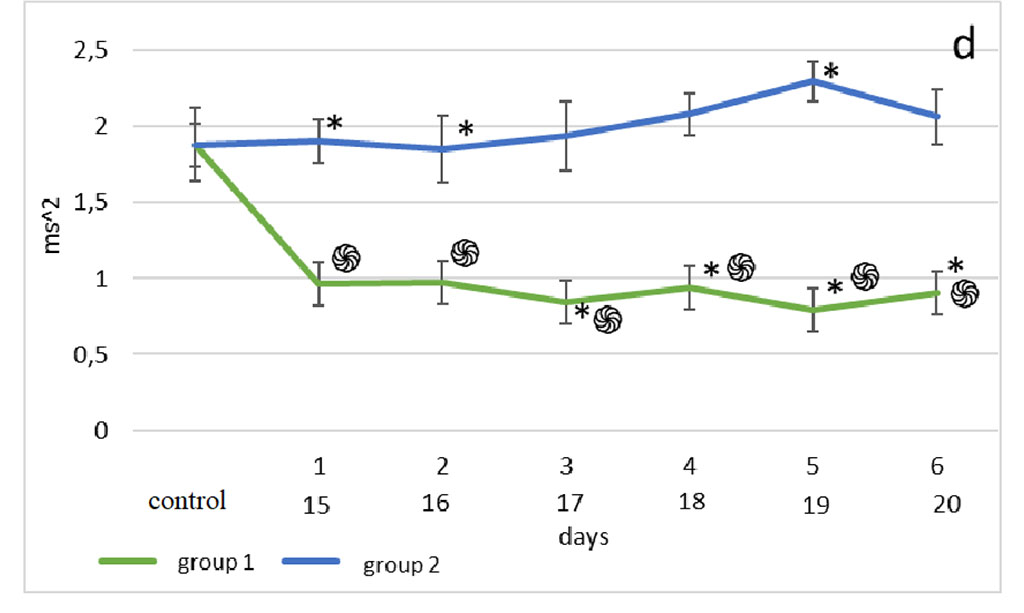
Figure 2. Changes in HRV frequency domain parameters after sensory deprivation simulation: LF (a), HF (b), VLF (c), LF/HF (d). Note: ֍ - significant differences between the experimental groups and the control group at p<0.001; • – significant differences between the experimental groups and the control group p<0.01; * - significant differences between the experimental groups at p<0.001; † – significant differences between the experimental groups at p<0.01; ‡ - significant differences between the experimental groups at p<0.05; ◊ - significant differences between the terms within the experimental groups; p<0.001 ○ - significant differences between the terms within the experimental groups p<0.01.
Compared with the 1st group, HF of the 2nd group was significantly higher than the 1st 2nd, 5th, 6th days (p<0.001), 3rd day (p<0.05), 4th day (p<0.01) (Fig. 2b).
The Mann-Whitney test showed that the very low-frequency component of the 2nd group was significantly higher than in the 1st group on days 1st-3rd, 5th-6th (p<0.001) and day 4th (p< 0.01). (Fig. 2c).
According to the Mann-Whitney test, one day after surgery, the highest LF/HF was observed in the animals of the 2nd group, compared with the 1st group and control (p<0.001). 48 hours after modeling the effects on the olfactory analyzer in group 2, a similar picture was observed (p<0.001), however, the lowest values of the vagosympathetic index in this segment of its assessment were noted in group 1. In the third observation period, the highest values were found in the 2nd group, compared with the 1st (p<0.001). At the 4th, 5th and 6th time points for assessing the vagosympathetic index, the situation was the same as in the previous period. But on the 5th day of observation, the LF/HF of the 2nd group was significantly higher than in the 1st (p<0.001) (Fig. 2d).
Surgical manipulations in the maxillofacial area lead to changes in heart rate variability [15].
Traditionally, frequency domain analysis of HRV reveals two or more peaks, a lower frequency (<0.15 Hz) and a higher frequency peak (>0.15 Hz), which are generally correlated with sympathetic and parasympathetic effects on cardiac activity, respectively. The decrease in HF compared to LF and the increase in VLF, which are observed simultaneously with changes in the behavior of rats under stress [8, 13], can be explained by the effect of post-surgical inflammation. High LF values in the bulbectomy group indicate a decrease in the activity of the SNS in the regulation of cardiac activity against the background of deprivation of the central part of the olfactory analyzer. It is known that fluctuations in the HF component are closely associated with the release of pro-inflammatory cytokines into the bloodstream an hour after exposure to a stress factor [11]. In addition, it has been shown that an anesthetic benefit that does not correspond to the volume of surgical intervention provokes vagotonia, which affects the regulation of cardiac activity [16]. In the early stages after septoplasty in the nasal cavity, inflammatory reactions are accompanied by mucosal oedema, and this leads to narrowing of the nasal passages and subsequent hypoxemia, which, in turn, increases PNS activity, which explains the increase in HF in the second group [15]. However, when modeling septoplasty in the first days after surgery, there was a drop in HF, probably due to the development of a classic stress response and the development of a depressive-like state. [17]. It is known that the VLF amplitude is closely related to emotional stress, and VLF can also show the regulation of metabolism [18]. The close connection of this HRV component with metabolism is confirmed by the relationship between diurnal changes in the concentration of the adipocyte hormone leptin in blood serum and diurnal changes in the VLF component of HRV [19]. The obtained difference between the groups can be explained by the fact that under conditions of complete deprivation of the olfactory analyzer, rats develop an anxiety state, and motor activity increases, since the olfactory analyzer in these animals is the leading one in cognition of the environment [8].
The vagosympathetic index (LF/HF) shows the ratio of the interaction between the SNS and PNS [20]. However, there is an opinion that this indicator does not quite accurately reflect the sympatho-vagal balance due to the fact that earlier many authors did not take into account its multifactorial nature of LF and HF [21]. There are other data showing that the vagosympathetic index can still reflect the state of balance of the autonomic nervous system. Thus, in fibromyalgia, an increase in LF/HF corresponds to a shift in the ANS to the dominance of the sympathetic division or to a decrease in the role of the parasympathetic, which is quite consistent with the nature of the autonomic function in fibromyalgia [22]. Septoplasty, in comparison with other models of operations in the maxillofacial region, in itself provokes an increase in the vagosympathetic index, which is due to the large area of the surgical field, a decrease in nasal passages, and sensory deprivation of the olfactory analyzer [1, 2, 7]. In the bulbectomy group, this figure is even higher, which can be explained by the complete absence of the function of the olfactory analyzer [8]. Previously, it was shown that modeling septoplasty in rats provokes the appearance of an anxiety-depressive state, which is manifested by changes in the behavior of animals [8, 23]. It is also known that surgical alteration in the maxillofacial region leads to changes in the cytoarchitectonics of the hippocampal pyramidal layer, an increase in neuronal apoptosis in the ammonium horn of the hippocampus [2], pronounced local inflammatory reactions of the depressive state, which is manifested by changes in the behavior of animals [15]. In addition, modeling of septoplasty in the early postoperative period provokes an increase in the activity of the sympathetic nervous system [8, 23], which is consistent with the data obtained in this study. Comparative characterization of the cytoarchitectonics of the hippocampus under conditions of sensory deprivation in various models remains to be studied [25-30].
Surgical traumatization of the nasal septum and upper jaw in rats in the early postoperative period causes a shift of the autonomic nervous system towards its sympathetic component. This indirectly indicates the occurrence of an acute stress response, the presence of a depressive-anxiety state, an increase in the mobilization of higher autonomic centers and an increase in the influence of neurohumoral and metabolic levels of regulation. The degree of stressfulness of bulbectomy in rats in the early postoperative period is more pronounced.
The authors declare no conflict of interest.