- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
The interest taken in studying pathomorphological and pathophysiological mechanisms behind periodontal inflammation affecting children with undifferentiated connective tissue dysplasia (UCTD) can be accounted for by the disease high prevalence, risk of its progression, as well as morphological and functional immaturity of tissues and low effectiveness of the available treatment options. The study into the matrix metalloproteinases (MMPs) expression in the gum and oral fluid biopsy material in children with UCTD is of both scientific and practical value in terms of diagnostic identification, disease course assessment, and the design of individualized treatment modes for periodontal diseases. In order to determine the specific features of MMP-2, MMP-8, MMP-9 expression, our study involved 61 children with periodontal diseases and various degrees of UCTD, as well as 27 healthy children with intact periodontium. The study implied analyzing the participants’ immune-histochemical studies of gum biopsy material and oral fluid enzyme immunoassay results. The enzyme immunoassay of MMP concentration in the oral fluid revealed that children with a prominent degree of UCTD, had their MMP-2, MMP-8, MMP-9 expression (medians 5.49 ng/ml, 478.4 ng/ml, 921.6 ng/ml, respectively) exceeding significantly the concentration of these enzymes in children whose degree of UCTD could be described as mild (medians 2.76 ng/ml, 317.1 ng/ml, 584.3 ng/ml, respectively).
An analysis of the MMP expression in the gum biopsy material showed that MMP-2 is not a reliable indicator to offer data required to assess the clinical status of periodontal tissues, since it is detected both in healthy children with intact periodontium and in children with periodontal disease and the UCTD syndrome. MMP-8 and MMP-9 can provide an objective description of the active phase of periodontium inflammation, which means these enzymes should be considered as reliable biomarkers of inflammation activity at the disease manifestation stage. Biochemical and immunohistochemical studies of MMP-2, MMP-8, MMP-9 the expression in oral fluid and gum biopsy material in children with UCTD syndrome should be used to identify the periodontium structural and functional status, the nature of inflammation, to detect potential risk groups vulnerable to the development of periodontal diseases, as well as to evaluate the effectiveness of various schemes for comprehensive treatment of periodontal issues.
Keywords: connective tissue dysplasia, matrix metalloproteinases, periodontal diseases, enzyme immunoassay, immunohistochemical examination, expression intensity, oral fluid, gum biopsy.
In the entire body of dental issues affecting children and adolescents, periodontal pathologies account for as much as 8.3%-88%, ranking second and giving way to dental caries only, whereas the rate of destructive types of periodontal diseases reaches 2-6%. Periodontal tissue diseases, which are to be viewed as both a general medical and a social issue, are the result of a whole set of local and general pathogenetic factors [1-5].
The main factors behind periodontal diseases in children include disturbed oral cavity microbiocenosis; immunological and neuroregulatory disturbances; altered hemodynamics, connective tissue metabolism, mineral metabolism; vitamin deficit; unhealthy nutrition; dental anomalies and deformities [6-12].
On the one hand, an imbalance among the oral cavity microbiocenosis elements leads to a decrease in the number of obligate microorganisms, while on the other hand, an increase in the level of potentially pathogenic and pathogenic bacteria comes forward as the leading factor promoting inflammation in periodontal tissues, which is further aggravated by a disturbance in the marginal periodontium microcirculation and by the involvement of immune mechanisms [13-15]. The developing inflammation turns to be chronic character over time, recurs and results in a destroyed dentoalveolar junction, destructed collagen fibers and connective tissue of the circular ligament, a developing periodontal pocket, alveolar bone tissue active resorption, and the destruction of the tooth supporting and retaining apparatus. The need to improve the comprehensive treatment schemes for periodontal diseases in children and adolescents is due to the morphological and functional immaturity of the main periodontal structures, the aggressive course of inflammation, the specifics of metabolism and pharmacokinetics typical of this age category, as well as resistance to traditional treatment measures [16-22].
Inflammatory periodontal diseases can show both as an isolated pathology and as part of multifactorial, environmental and genetic diseases and syndromes. Periodontal pathology is especially common in case of undifferentiated types of connective tissue dysplasia (UCTD). The growing interest taken lately in UCTD affecting children and adolescents is due to the following factors: wide prevalence (individual signs occur in 14-85%); polymorphism of clinical and morphological disorders; polyorganic and polysystemic nature of lesions; disease course progression; biochemistry and physiology involvement; high risk of developing associated (secondary) pathological conditions affecting inner organs and systems; lack of commonly accepted diagnostic criteria; difficulties in assessing the nature, number and specificity of symptoms; specific features of the clinical course; negative impact the syndrome has on the course, prognosis and treatment of various diseases. In case of UCTD, significantly affected are the organs and systems that feature a high content of collagen: the bone-and-joint system, the cardiovascular system, the respiratory system, the organs of vision, the dental system [23-31].
A study into the matrix metalloproteinase system (MMP) components proved their diagnostic and prognostic value in case of a variety of diseases involving the cardiovascular, immune, bone and joint systems, as well as periodontal pathology. MMPs belong to Zn2+ and Ca2+-dependent endopeptidases, which are degradation mediators for all types of collagens and other components of the extracellular matrix (elastin, proteoglycans, laminin), whose synthesis and accumulation play a key role in the basement membrane hydrolysis. Extracellular matrix not only plays a supporting role for organs and tissues, yet is also involved actively in other functions, namely, cell cycle and cell mobility regulation, survival and apoptosis, as well as biologically active molecule distribution and intercellular interaction. In healthy tissues, MMPs are involved in processing extracellular matrix proteins, also playing a key role in physiological processes like embryogenesis; morphogenesis; neoangiogenesis; resorption and cell matrix remodeling; migration, adhesion, differentiation and proliferation of cells, and tissue repair. In case of pathologies, excessive MMP activity leads to excessive destruction of extracellular tissue elements, thus resulting in disrupted functioning of cellular structures [32-36].
Speaking of proteolytic degradation of the periodontal connective tissue extracellular matrix and the maintenance of respective inflammation, a significant role resides here in gelatinases MMP-2, MMP-9 and collagenase MMP-8. The highest level of proteolytic activity in relation to collagen types I, II, III can be observed in neutrophil collagenase (MMP-8), which is a biomarker of extracellular matrix progressive destruction in inflammatory periodontal pathology. MMP-2 plays an important role in the degradation of denatured collagen and type IV collagen, as well as it tends to increase along with the progression of inflammation affecting the periodontium. A significant contribution in collagenolysis is also made by gelatinases (MMP-2 and MMP-9), whose main substrate is type IV, V collagen, denatured collagen, and laminin. The wide substrate specifics of MMPs is decisive in their leading role not only in the extracellular matrix destruction affecting periodontium connective tissue, but also in maintaining (modulating) the inflammatory process that is underway there [37-39].
An analysis of the respective literature shows that in order to ensure reliable assessment of the degree of periodontal diseases as well as to forecast the development of potential groups highly vulnerable to periodontal disease among children with UCTD syndrome, apart from clinical, anthropometric, X-ray and functional studies, advanced non-invasive molecular biochemical and immunohistochemical research methods appear of diagnostic significance [40-47]. Studying the specific features of MMP expression in the gum biopsy material and oral fluid in children with UCTD syndrome and periodontal diseases will help arrive at insight into the mechanisms of periodontal diseases etiopathogenesis; enhance the reliability of non-invasive methods employed to evaluate the status and the activity of proteolytic processes in periodontium, and identify the nature of the disease course at the stage of primary diagnostics and monitoring thus helping develop a pathogenetically reasonable treatment to be offered to this category of patients.
Aim of study – to identify the specific features of MMP-2, MMP-8, MMP-9 informativeness in the gum biopsy material and oral fluid in children with UCTD syndrome and periodontal diseases employing immunohistochemical and enzyme immunoassay methods.
The study involved 88 children (51 girls, 37 boys) aged 12-17. The inclusion criteria (n=61; 37 girls, 24 boys) for the main group were: age – 12-17; mild gingivitis (PMA – below 30%); good (OHI-S – under 0.6) and average (OHI-S – 0.7-1.6) oral hygiene; clinical symptoms of UCTD. The diagnosis was verified subject to the Russian recommendations «Hereditary and multifactorial connective tissue disorders in children. Diagnostic algorithms» (2014). The severity of the dysplastic process was assessed in view of the dysplasia diagnostic criteria of by L.N. Abbakumova (2006) and the scoring scale for assessing the significance of phenotypic signs (Table 1).
Table 1. Score assessment of phenotypic trait significance in assessing the connective tissue dysplasia severity by L. N. Abbakumova (2006)
| Sign | Score | Sign | Score |
| Eye hypertelorism | 1 | Incomplete syndactyly of 1st and 2nd toes | 2 |
| Blue sclera | 1 | Dilated capillaries of skin of face, back | 2 |
| Wide nose bridge | 1 | Scars on skin | 2 |
| Ingrown ear lobes | 1 | High palate | 3 |
| Pigmented spots | 1 | Increased skin extensibility | 3 |
| Asthenic physique | 1 | Pronounced venous skin pattern | 3 |
| Clinodactyly of little fingers | 1 | Easy development of hematomas | 3 |
| Epicanthus | 2 | Hernias | 3 |
| Saddle-shaped nose | 2 | Weakness of abdominal muscles | 3 |
| Protruding ears | 2 | Transverse striation of feet | 3 |
| Asymmetry of nasal septum | 2 | Flat feet | 3 |
| Pallor of skin | 2 | Hallux valgus | 3 |
| “Suede-like” skin | 2 | Hollow foot | 3 |
| Delicate skin | 2 | Eyesight pathology | 4 |
| Wrinkled skin | 2 | Pronounced hypermobility of joints | 4 |
| Flat chest | 2 | Kyphosis | 4 |
| Slight indentation on chest | 2 | Scoliosis | 4 |
| Clavuses | 2 | Keeled thorax | 5 |
| Sandal-shaped slit | 2 | Funnel-shaped deformation of chest | 6 |
The total score in children with a mild degree of UCTD did not exceed 12; in case of a moderate degree of CTD – 13-23, whereas cases with a prominent degree of CTD had a score of 24 or beyond. Details concerning the phenotypic manifestations of UCTD were obtained from the medical records, medical reports of respective experts, anamnestic data (anamnesis morbi, anamnesis vitae). Depending on the phenotypic manifestation severity, the children were divided into two subgroups: Subgroup 1 were children (n=32) with a mild and moderate degree of UCTD; Subgroup 2 included children (n=29) with a prominent degree of UCTD. The comparison group included children (n=27; 14 girls, 13 boys) aged 12-17 who belonged to Health Groups I, II, with clinically intact periodontium and good (OHI-S – less than 0.6) oral hygiene. All the patients’ parents signed informed consent agreeing to join the study. Clinical, biochemical and morphological research methods were subject to the applicable ethical principles for medical research involving humans as claimed by the World Medical Association Declaration of Helsinki (1964, 2000). The periodontological and hygienic status was assessed employing the papillary-marginal-alveolar index (PMA) (Schour, Massler 1947) Parma modification (1960), and the simplified Green-Vermillion Hygiene Index (OHI-S) (Green, Vermillion, 1964). The diagnosis was in view of the entire body of clinical and radiological signs, relying on the Classification of periodontal diseases (2001).
Unstimulated oral fluid (UOF) sampled to identify the MMP level was obtained following the rules below: abstinence from any meal for at least an hour; preliminary double rinsing with distilled water (t = 20-25°); after swallowing saliva, the child tilted the head over a funnel, which let the UOF flow freely into a sterile tube; as soon 2.0 ml of UOF obtained (collection time – no more than 15 min), the procedure was terminated, with the tube was hermetically sealed with a stopper and placed in an ice container to be further delivered to a biochemical laboratory. Then, the UOF was poured into aliquots of 250-300 µl in sterile plastic tubes and stored at t = -80° C until the study was carried out (Fig. 1).
Fig. 1. Frozen oral fluid
The aliquots containing UOF were then defrosted and centrifuged (at 10,000 rpm, for 2 minutes) on a Mikro 185 (Hettich) microcentrifuge. The MMP content in the supernatant fluid was detected through a direct enzyme immunoassay, taking into account the manufacturer’s instruction, using the following standard reagent kits: Human/Mouse/Rat MMP-2 (total); Human MMP-8 (total) ELISA; Human MMP-9 (total) ELISA (Quantikine ®, R&D Systems, USA). The studies were carried out on an ImmunoChem-2100 (High Technology Inc) multifunctional microplate reader for enzyme immunoassays (Fig. 2).

Fig. 2. An ImmunoChem-2100 microplate photometer
The optical density was measured at λ = 450 nm (correction at λ =540 nm). The results were calcu-lated based on the following formula: Y = a + bX + cX2; where Y is the optical density, X is the concentra-tion of the proteinase analyzed (ng/ml).
The gum biopsy material for immunohistochemical examination obtained from the main group, was sampled from spots featuring the most obvious inflammation, whereas in case of patients with intact periodontium, it was obtained from the gum at the teeth to be removed for orthodontic reasons. Immunohistochemical studies to detect MMP-2, MMP-8 and MMP-9 in gum tissues were carried out subject to the standard protocol, while using a Thermo Scientific Autostainer 480S device for automated immunohistochemical staining and a PT Module, Thermo Scientific water bath used to dewax drugs and unmask antigens (Fig. 3).

Fig. 3. A Thermo Scientific Autostainer 480S device for automatic immunohistochemical staining with a 2D Barcode system and a PT Module, Thermo Scientific water bath device for dewaxing drugs and unmasking antigens (Thermo Fisher Scientific).
The biopsies were fixed in a 10% formalin solution buffered by Lilly (pH – 7.4). To detect the MMP expression, paraffin sections (thickness - 5 microns) were used, which were applied to salinized object-plates (Daco). Dewaxing was performed for 1 hour in a thermostat at +60° C. After immersion in xylene, the slices were rehydrated by taking them through alcohols with decreasing concentration for 15 minutes. The antigenic activity was recovered by thermal unmasking in a 0.01M citrate buffer in a Return-2100 mini-autoclave. Endogenous peroxidase activity was blocked by a 0.3% Н2О2 solution (10 min). The following antibodies to human antigens were used: rabbit polyclonal antibodies to MMP-2 (polyclonal rabbit, Epitomics, 1:100); rabbit monoclonal antibodies to MMP-8 (monoclonal rabbit, Gene Tex, 1:100); rabbit polyclonal antibodies to MMP-9 (polyclonal rabbit, Thermo Fisher Scientific, 1:100).
During the immunohistochemical reaction, 50 µl of diluted primary serum was applied to the slices. The slices were incubated for 30 minutes at +37°C. The immune complexes were identified with a BioGenex (USA) biotin-free detection system based on horseradish peroxidase. Following each incubation, the slices were washed in a phosphate-salt buffer and dried, which was followed by the application of 3,3-diaminobenzidine to evaluate the reaction, which was identified by the presence of granules of a specific brown color. Once arriving at the required staining intensity, the sections were washed in distilled water and finished with Mayer’s hematoxylin. A positive reaction to MMP manifested itself as cytoplasm brown staining of varying intensity. The MMP expression intensity was evaluated in view of the following criteria: weak – under 10% of weakly colored (+) cells; moderate − 10-30% of moderately colored (++) cells; strong − above 30% of intensely colored (+++) cells. The objects were photographed using an Axio Imager 2 binocular microscope (Carl Zeiss) with an AxioCam ICc 5 digital video camera (Carl Zeiss) (Fig. 4).

Fig. 4. An Axio Imager 2 binocular microscope with an AxioCam ICc 5 digital camera.
The statistical data processing was performed using IBM SPSS Statistics 24.0 software in Windows 10 environment, with the median and the interquartile intervals (Me (Q25; Q75)) calculated for signs featuring a distribution different from normal, while there were also nonparametric methods of comparing unrelated features used – the Kruskal-Wallis analysis in case of the number of groups to be compared exceeding two; the Mann-Whitney (U) criterion – when comparing two groups. The Wilcoxon (T) criterion was used for related values. In case of features with a normal distribution, the mean ± standard deviation (M±SD) was calculated, as well as parametric methods of comparing unrelated features were used – one-factor variance analysis with Bonferroni correction when comparing more than two groups, and Student’s t-test – for two groups. The differences were considered statistically significant at p<0.05.
Figures 5-8 show the details of the dental status in the studied groups
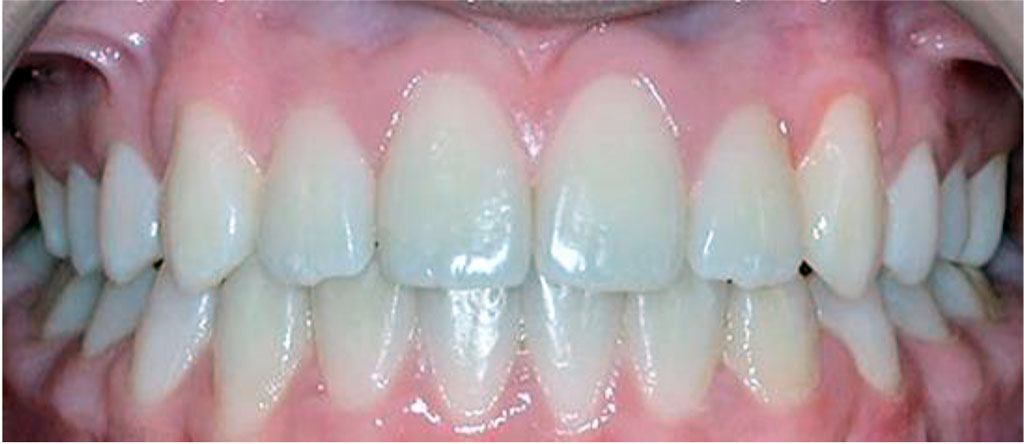
Figure 5. Patient K., 17 y.o. (comparison group). Clinically healthy periodontium and a “good” (OHI-S = 0.4) level of oral hygiene.
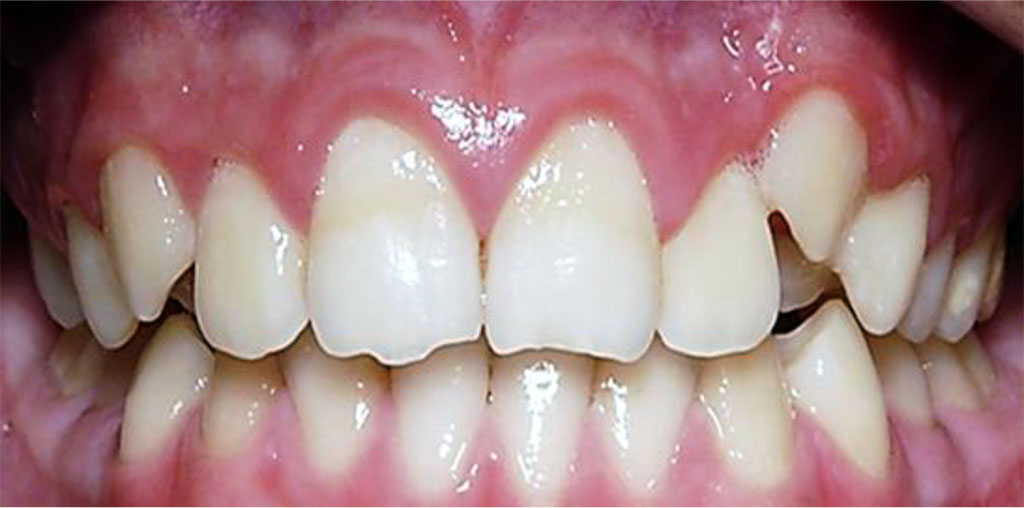
Figure 6. Patient P., 14 y.o. (mild UCTD; points scored – 11 (L.N. Abbakumova, 2006)). Ds: chronic gingivitis (catarrhal) K05.1 (ICD-10) and moderate (PMA index – 32.4 %), oral hygiene – satisfactory (OHI-S = 0.9).
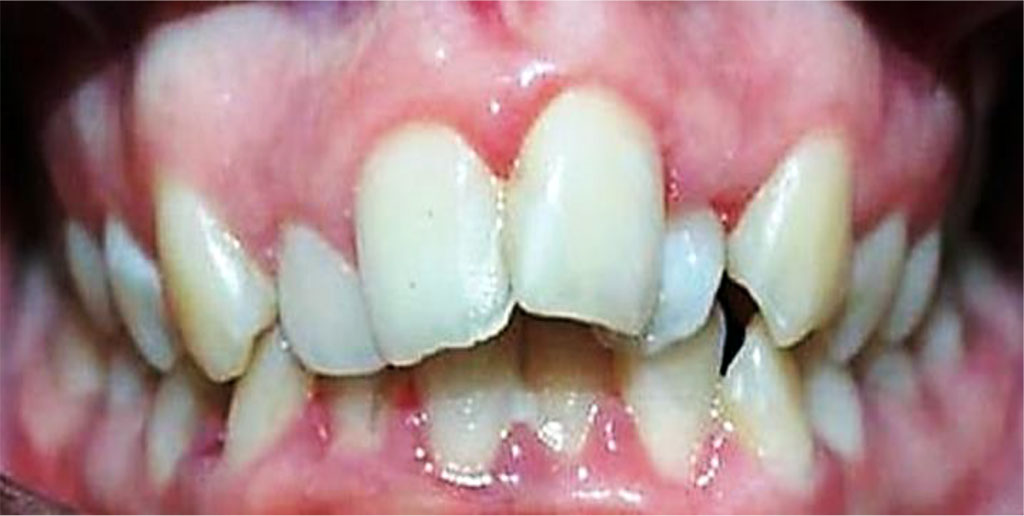
Figure 7. Patient А., 16 y.o. (moderate UCTD; points scored – 19 (L.N. Abbakumova, 2006)). Ds: chronic gingivitis (catarrhal) K05.1 (ICD-10) and moderate (PMA index – 35.8 %), oral hygiene – satisfactory (OHI-S = 0.9).
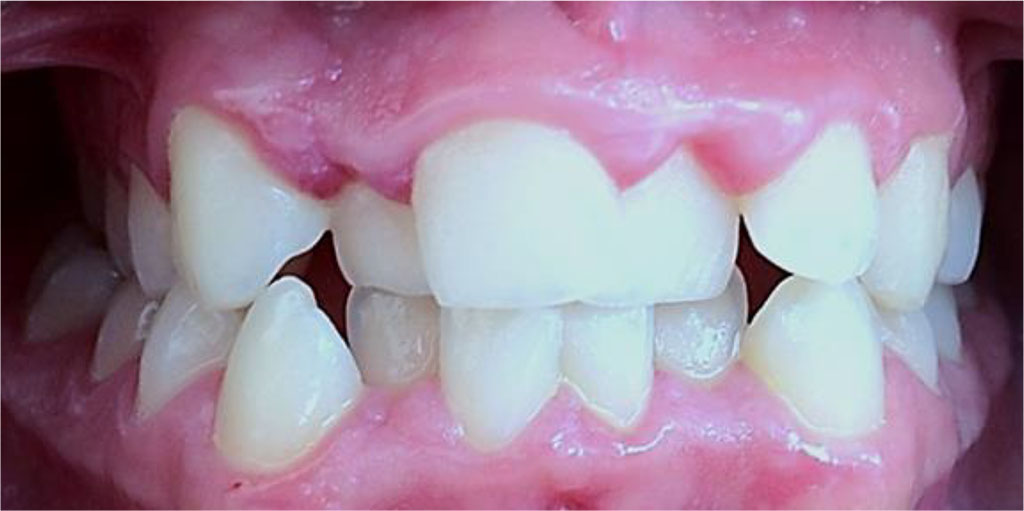
Figure 8. Patient B., 15 y.o., significant degree of UCTD; points scored – 25 (L.N. Abbakumova, 2006)). Ds: chronic gingivitis (catarrhal and hypertrophic) K05.1 (ICD-10) moderately severe (PMA – 43.9 %), oral hygiene – satisfactory (OHI-S = 1,1).
The results of assessing MMP expression through immunohistochemical studies reveal that it is only MMP-2 that is detected in children with intact periodontium, MMP-8, MMP-9 not identified. Children with healthy periodontium in all cases (n=27; 100%) were found to have a weak (+) expression of fibroblast secreted MMP-2, which plays an important role in the degradation of denatured collagen and collagen types I-IV (Fig. 9).
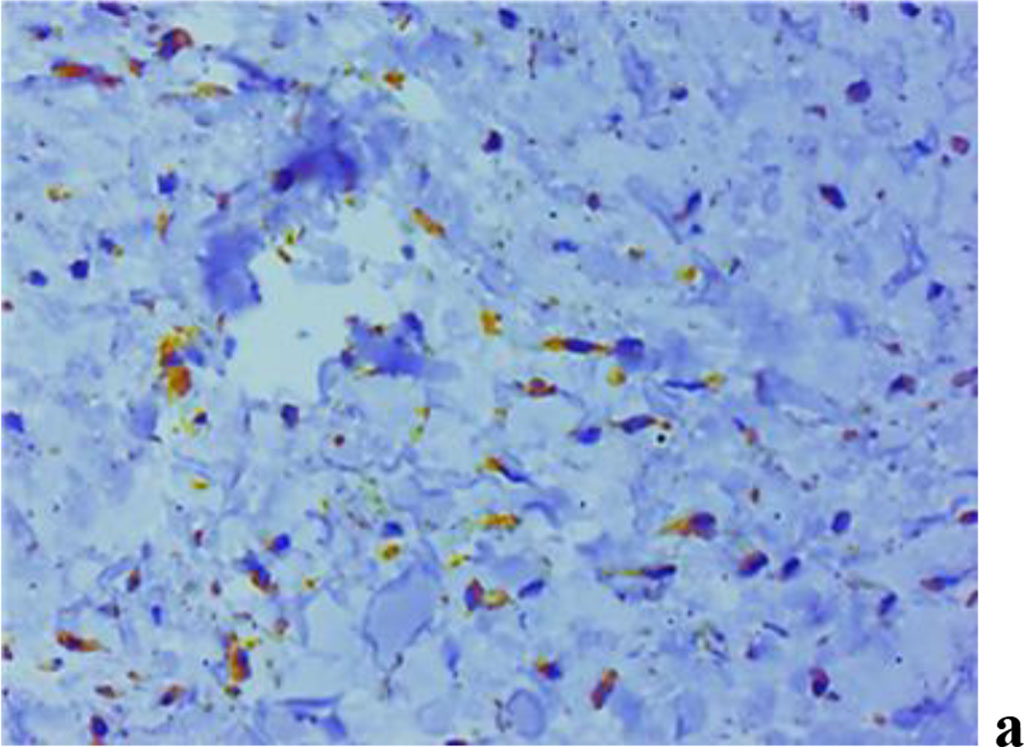
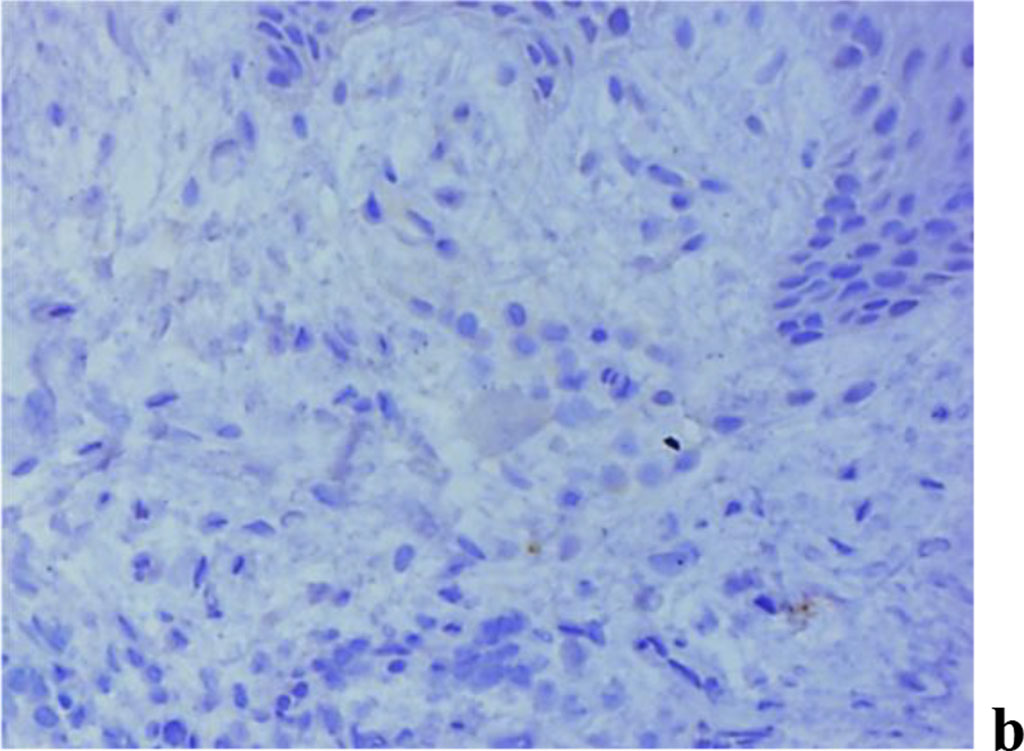
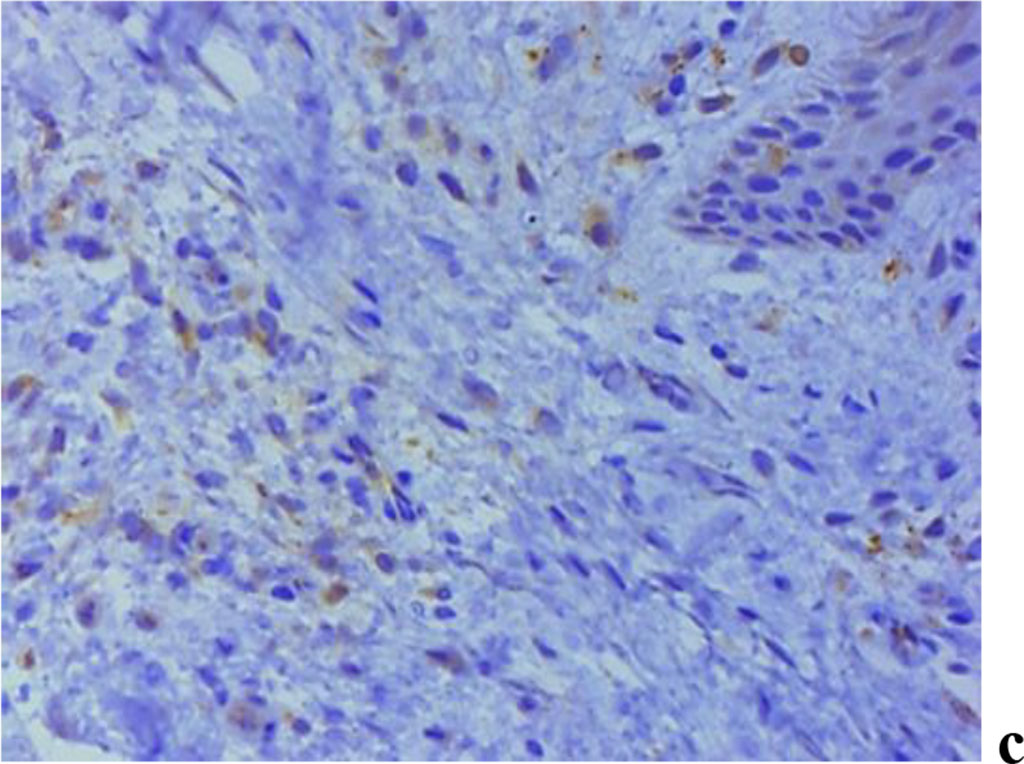
Figure 9. Immunohistochemical staining with antibodies to MMP-2(a), MMP-8(b), MMP-9 (c) in gum biopsy material in children with intact periodontium with no clinical symptoms of UCTD; ×500 (chromogen − DAB, Mayer hematoxylin counterstaining).
A study into the nature of MMP expression performed by the immunohistochemical method, revealed that both MMP-2 and MMP-8, MMP-9 are to be observed in children with inflammatory periodontal diseases and a mild degree of UCTD (Fig. 10).
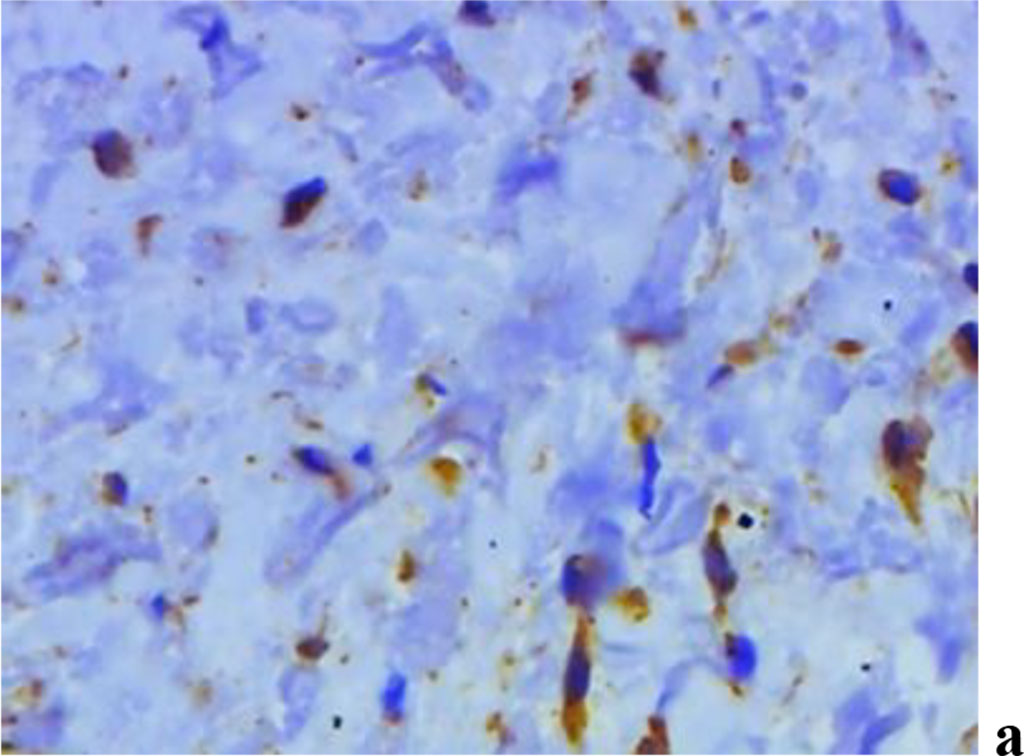
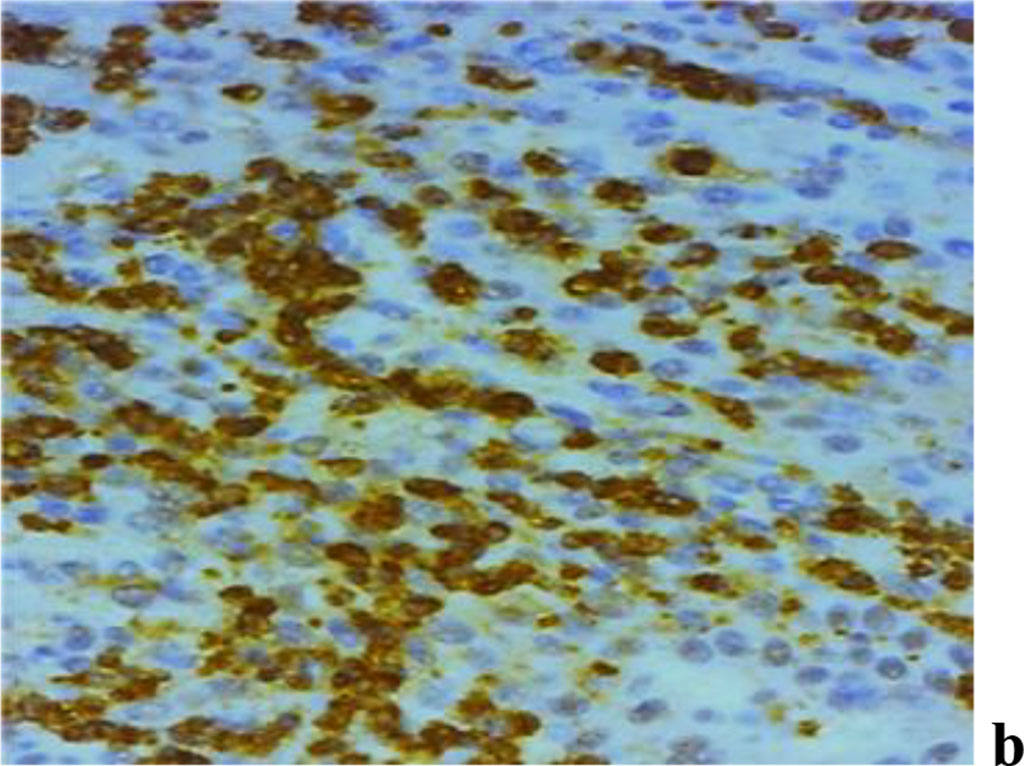
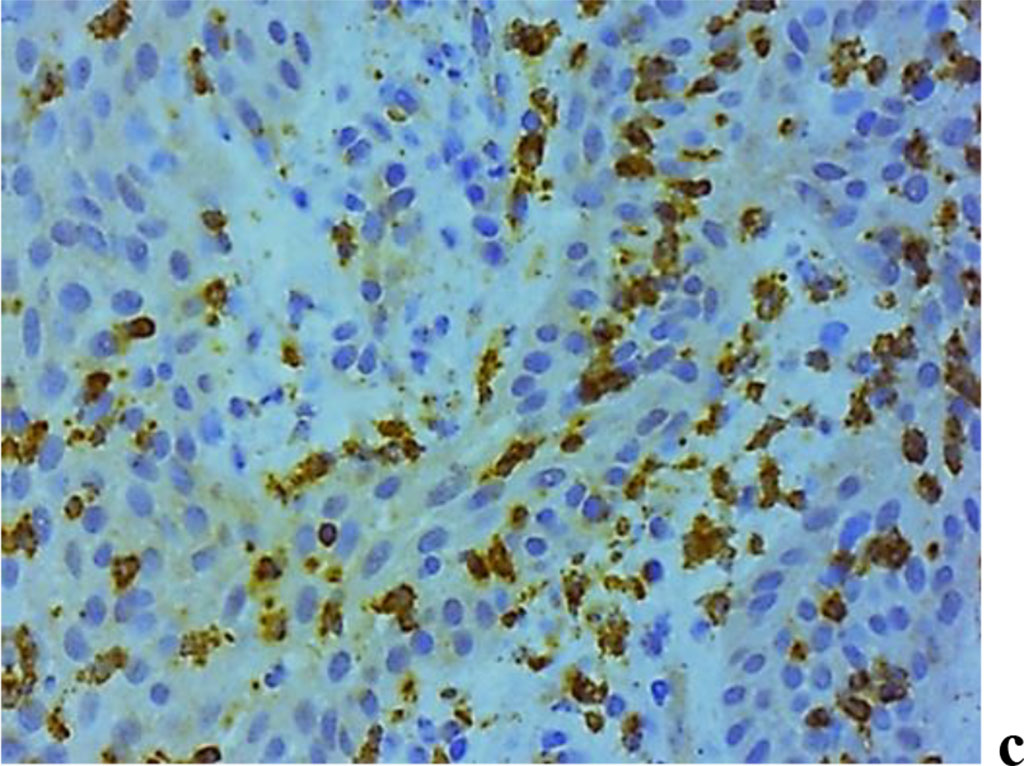
Figure 10. Immunohistochemical staining with antibodies to MMP-2(a), MMP-8(b), MMP-9 (c) in the gum biopsy material in children with inflammatory periodontal pathology and mild UCTD; ×500 (chromogen − DAB, Mayer hematoxylin counterstaining).
The children in the main group (subgroup 1; n=32), as the immunohistochemical studies of the gum biopsy material revealed, had the following morphological features: weak (+) expression of MMP-2 − in 90.7% of cases (n=29); moderate (++) expression of MMP-2 − in 9.3% of cases (n=3); weak (+) expression of MMP-8 − in 71.9% of cases (n=23), moderate (++) expression of MMP-8 − in 28.1% of cases (n=9); weak (+) expression of MMP-9 − in 78.1% of cases (n=25), moderate (++) expression of MMP-9 − in 21.9% of cases (n=7).
The analysis of MMP expression, performed with immunohistochemical tests suggests that MMP-2, MMP-8 and MMP-9 could be identified in children with periodontal inflammatory pathology and a significant degree of UCTD (Fig. 11).
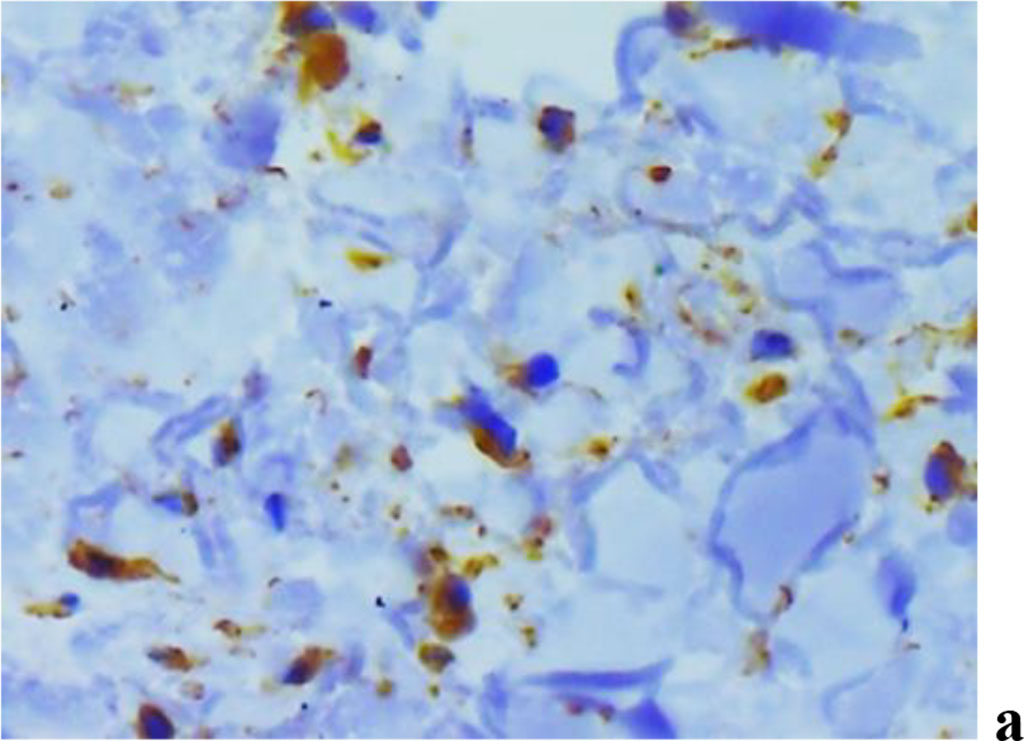
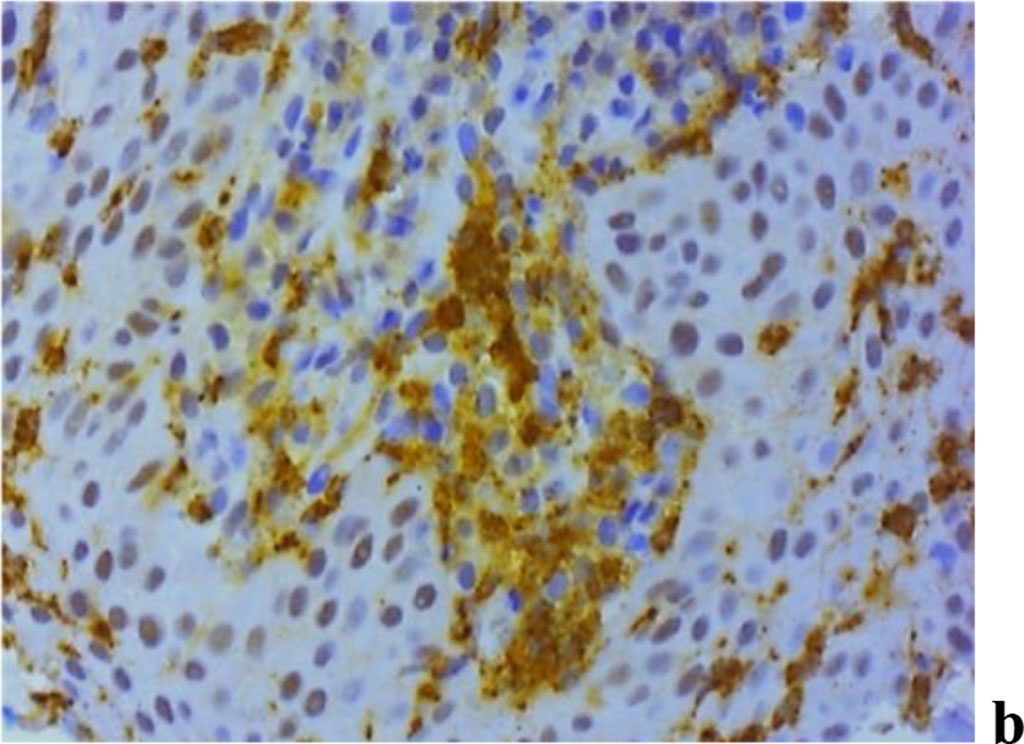
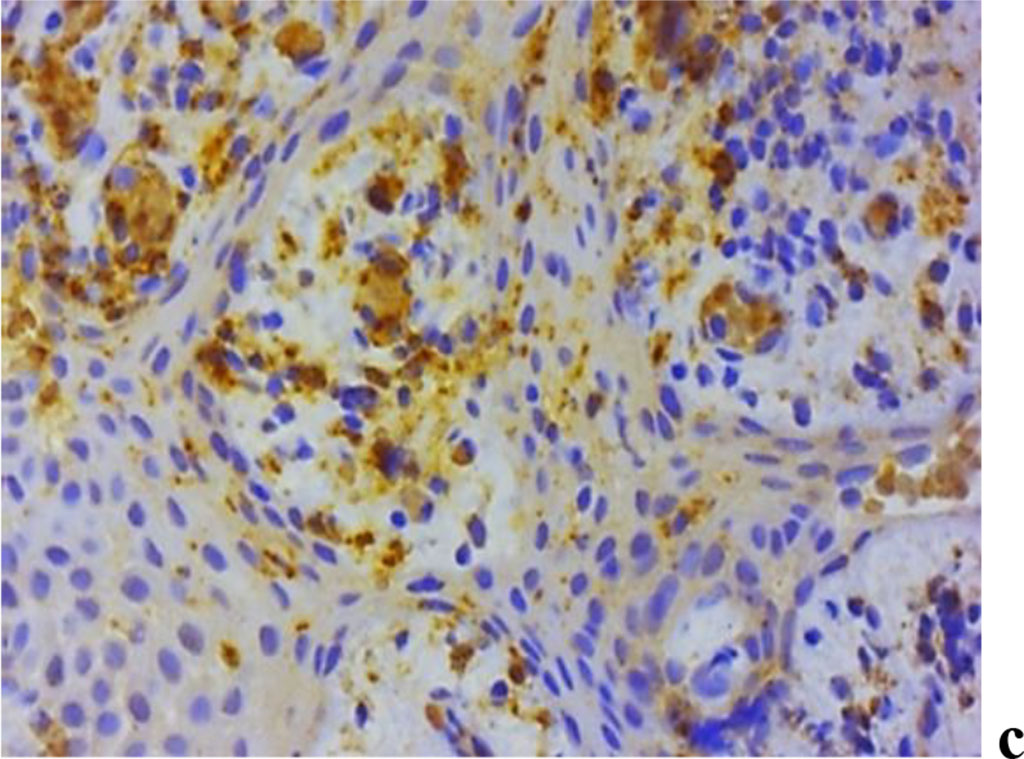
Figure 11. Immunohistochemical staining with antibodies to MMP-2(a), MMP-8(b), MMP-9 (c) in the gum biopsy material obtained from children with inflammatory periodontal pathology and mild UCTD; ×500 (chromogen − DAB, Mayer hematoxylin counterstaining).
In subgroup 2 of the main group (n=29), as immunohistochemical studies of the gum biopsy material suggest, the following morphological features were identified: weak (+) expression of MMP-2 − in 27.6% of cases (n=8); moderate (++) expression of MMP-2 − in 55.2% of cases (n=16); strong (+++) expression of MMP-2 − in 17.2% of cases (n=5); weak (+) expression of MMP-8 − in 6.9% of cases (n=2); moderate (++) expression of MMP-8 − in 51.7% of cases (n=15); strong (+++) expression of MMP-8 − in 41.4% of cases (n=12); weak (+) expression of MMP-9 − in 17.2% of cases (n=5); moderate (++) expression of MMP-9 − in 62.1% of cases (n=18); strong (+++) expression of MMP-9 − in 20.7% of cases (n=6).
Figure 12 offers a look at MMP expression in biopsies of gum material obtained from the groups included into the study.
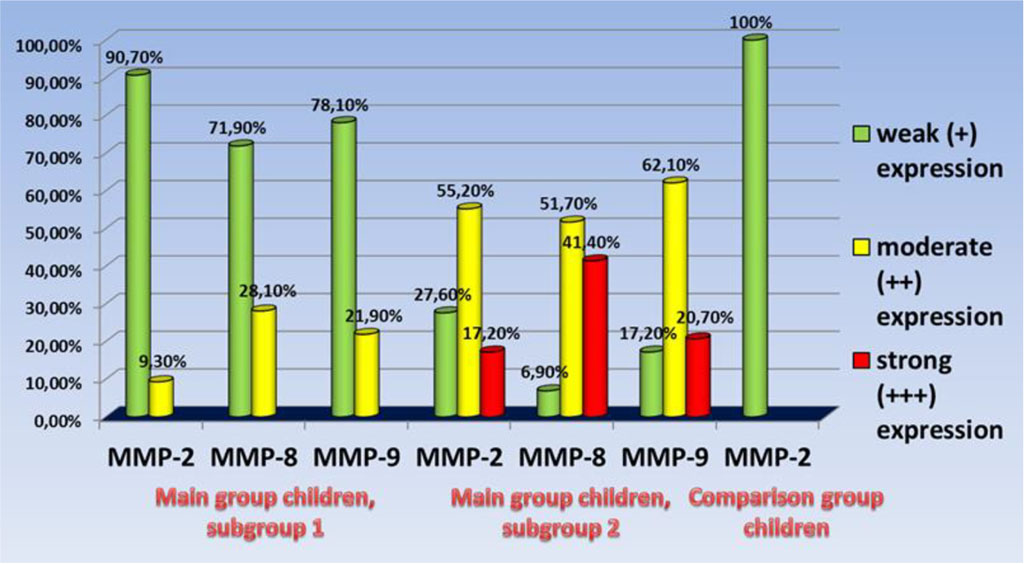
Figure 12. The intensity of MMP expression in the biopsy material of the gums of the children of the studied groups.
The assessment results of MMP expression intensity in the gum biopsy material observed in the main group reveal that when talking about the pathogenesis of inflammatory periodontal diseases in children with UCTD syndrome, a higher level of MMP-8 expression is of the greatest importance, compared with a lower level of MMP-9 expression. The children in subgroup 1, therefore, featured moderate expression of MMP-8 in 28.1% of cases, while for MMP-9 expression the same rate was 21.9% of cases; in subgroup 2, though, strong expression of MMP−8 was to be observed in 41.4% of cases, and MMP-9 – in 20.7% of cases, respectively. The said trend identified for MMP-8, MMP-9 expression at the stage of periodontal disease manifestation well matches the research data that is available in published sources [48-50].
Table 2 offers details of the MMP contents in UOF in the studied groups
Table 2. MMP-2, MMP-8, MMP-9 content in oral fluid (children, studied groups) (Me (25; 75 %)), (ng/ml)
| Indicator | Healthy children | Children with mild, moderate UCTD | Children with significant UCTD | p |
| MMP-2 | 0.02 (0.0; 1.64) | 2.76 (0.34; 5.83) | 5.49 (1.78; 12.06) | 0.028 |
| MMP-8 | 203.8 (127.6; 314.9) | 317.1 (168.6; 522.7) | 478.4 (143.8; 707.2) | 0.016 |
| MMP-9 | 366.2 (196.4; 538.5) | 584.3 (271.8; 1086.3) | 921.6 (365.4; 1517.1) | 0.034 |
The enzyme immunoassay analysis focusing on the MMP content in UOF in the groups of children described above shows that the salivary level of MMP-2, MMP-8 and MMP-9 in children with a significant degree of UCTD (medians 5.49 ng/ml, 478.4 ng/ml, 921.6 ng/ml, respectively) is statistically (p<0.05) above similar indicators observed in patients with a mild (moderate) degree of UCTD (medians 2.76 ng/ml, 317.1 ng/ml, 584.3 ng/ml, respectively) and in children belonging to health groups I and II (medians 0.02 ng/ml, 203.8 ng/ml, 366.2 ng/ml, respectively).