- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
The purpose of this experimental work was to determine the regeneration ability of bone tissue due to modern bioceramics synthesized and obtained using the previously developed method of reactive spark plasma sintering (Spark Plasma Sintering-Reactive Synthesis, SPS-RS). The study was performed on 26 laboratory rabbits - females of the New Zealand White breed, divided into two groups. The evaluation of the regeneration of the burr hole after covering the defect with a bioceramic implant was performed by the method - (BMD) X-ray densitometry on days 7, 40, 210 after surgery. As a result of the study, there was a tight fixation of the implant in the area of the bone tissue defect without its dislocation. There were no signs of osteoporosis and osteolysis in the surrounding bone tissues. And on the 210th day after the operation, according to densitometric data, the formation of dense bone tissue was observed. The use of a bioceramic implant makes it possible to immediately close large bone defects and to stimulate the formation of dense bone structures around them.
Keywords: Bioceramic implants, Bone tissue regeneration, osteolysis, implant based on zirconium oxide, bone defects, osteoporosis.
The problem of bone tissue regeneration and closure of bone defects is relevant in modern surgery. The number of patients with bone defects after resection of bone neoplasms due to fractures, chronic infection is rapidly increasing. Thus, more than 1 million bone grafts are performed annually in the European Union [1]. Autograft is the gold standard for treating bone defects. However, the scarcity of donor sites limits the number of autografts obtained from both the iliac crest and other parts of the patient's body. In addition, during explantation, additional trauma is applied, nerve damage, the appearance of a pronounced pain syndrome, and the occurrence of new fractures are possible [2,3,4,5].
The medical use of xenografts (eg, bovine bone) is usually associated with potential viral infections. In addition, xenografts have low osteogenicity, increased immunogenicity and, as a rule, resolve faster than autologous bone [6,7,8,9]. Similar restrictions are also valid for human allografts when the risks of tumor cells, various bacterial and viral infections, and immunological incompatibility remain. In addition, extraction and preservation of allografts (exogenous bones) are additional limiting factors. [10,11,12].
Various biomaterials for bone regeneration are currently developed, such as bone collagen, decalcified bone matrix, fibrin, nano-hydroxyapatite/collagen, synthetic polymer (glycolic co-lactic acid), real bone ceramic or sintered bovine bone and titanium [13,14,15,16,17]. Each material has its advantages, while at the same time many disadvantages. Artificial materials (alloplastic or synthetic bone grafts) are promising. They are readily available and can be processed and modified to meet the specific needs of a particular application. Moreover, there are no concerns about potential infections, immunological incompatibility, sterility and morbidity of the donor site. Therefore, studies of artificial materials for bone tissue restoration are promising in the field of research on biomaterials for clinical use [18,19].
The study used 26 clinically healthy vaccinated rabbits - females of the New Zealand White breed weighing from 4.5 to 5.2 kg, aged from 1.5 to 1.7 years. The study was carried out in accordance with the European Union directive on the protection of animals used for scientific purposes (2010/63/EU), in accordance with the law on the protection of animals from violence (Chapter 5, asset 104679-GD of 01.12.1999), Declaration of Helsinki of 1975 and its edition since 2000, as well as the rules for handling and caring for laboratory animals in accordance with GOST 33215–2014 “Rules for equipping premises and organizing procedures for working with laboratory animals”, SanPiN 2.2.1.3218–14 - “Sanitary and epidemiological requirements for the arrangement, equipment and maintenance of experimental biological clinics and vivariums”, GOST 33216–2014 - “Rules for laboratory work with rodents and rabbits”. The design and protocol of the study was approved by the Interdisciplinary Ethics Committee of the Pacific State Medical University No. 3 dated November 16, 2020.
All animals were operated on in an experimental operating room in compliance with the rules of asepsis and antisepsis. During surgery, intramuscular anesthesia with sodium pentobarbital (25 mg/kg) and xylazine (8 mg/kg) for pain relief was administered to each rabbit prior to surgery. During the operation, the animals were shaved a 30 x 30 mm section of skin on the head in the projection of the parietal bone. After treatment with a solution of the skin antiseptic Betadine 10%, with a scalpel No. 15 up to 3 cm long, a linear skin incision was made according to the preliminary operational marking, the tendon helmet was dissected and the frontal bone was exposed. Using an electric cutter with a diameter of 2.5 mm, a burr hole with a diameter of 10 mm was formed under aseptic conditions. In order to prevent secondary bone damage due to excessive temperature rise, saline cooling was performed. Bleeding was stopped using an EHVCh-80-03-FOTEK coagulator in the standard electrocoagulation mode with a power of 10 W. The main group consisted of 19 animals, which were implanted with cylindrical ZrO2-(15 mas.%)HAp (doped with hydroxyapatite) into the formed bone defect. The implants were fixed by suturing the tendon helmet with atraumatic absorbable suture material Vicryl 4.0 (Johnson & Johnson USA), without additional fixation to the bone tissue. The control group included 7 animals, in which the bone defect was not closed with a ceramic implant after the operation. The skin of all animals was sutured with atraumatic nonabsorbable monofilament material Prolene 3.0 (Ethicon, USA). The sutures were removed on the 14th day. day.
Animals were observed once a day immediately after surgery, and then 3-4 times a week to determine their condition, the presence of complications directly related to surgery.
In the study, samples of ceramic materials with a size of 8 × 7 × 3 mm were used for implantation, obtained by a previously developed method of reactive spark plasma sintering (Spark Plasma Sintering-Reactive Synthesis, SPS-RS). The resulting composite bioceramic contains 20 wt.% hydroxyapatite, has high porosity (Ssp=8.56-12.7 m2/g) with pore sizes in a wide range from 1 to 100 µm, has good structural and mechanical characteristics: high compressive density - 560 MPa and density 2.75-2.86 g/cm3. Instrumental research methods. The dynamics of the regenerative process of bone tissue in the region of the skull defect was studied by calculating bone mineral density (BMD) by X-ray densitometry. An Agulion Toshiba (Japan) multispiral 64-slice CT scanner was used under native imaging conditions at a voltage of 120 kV and a current of 50 mA, with a collimation of 0.5 mm, and a subsequent reconstruction interval of 0.3 mm. 3-D reconstruction was carried out using the original program Agulion "Toshiba" (Japan). The study was carried out on the basis of the Center for Collective Use "Far Eastern Center for Non-Invasive Radiofrequency Diagnostics of Biological Objects" (CCU FERRD).
Before the operation, the skull bones were examined, the thickness of the cortical plate of the parietal bone was measured, and bone density was measured in the area of the proposed surgical intervention. To ensure measurement accuracy, the animals were sedated by intramuscular injection of a 0.5 mg/kg solution of acepromazine. The average value of bone density in the area of the proposed surgical intervention in intact rabbits was 773.4±28.6 HU. According to MSCT data, on the 3rd day after implantation of the ZrO2-(15 wt.%) HAp ceramic bioimplant, it was revealed that a cylindrical implant of high densitometric density (+20043 HU) was installed in the defect from the burr hole of the parietal bone of the rabbit skull. In all cases, we revealed a high degree of congruence between the implant and the burr hole. The brain tissues of the animals remained intact, there were no CT signs of edema of the brain substance and displacement of the median structures of the brain. The implants were covered with a sutured tendon helmet and soft tissues. Edema was determined only in soft tissues as a result of trauma during surgery (Figure 1).
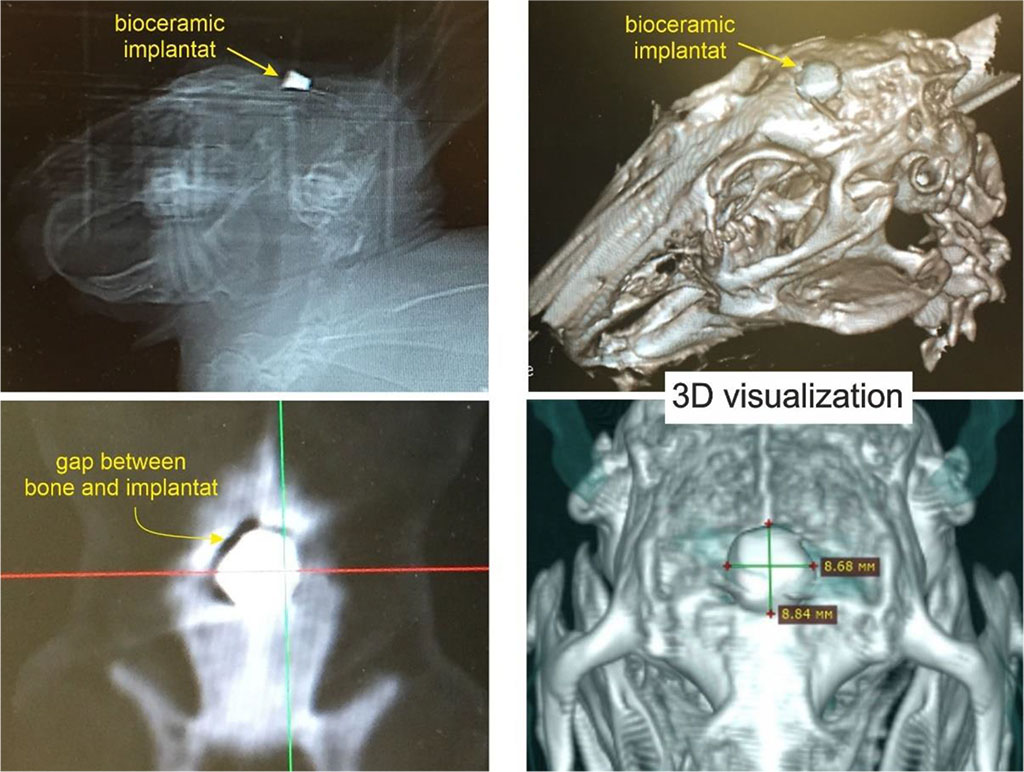
Figure 1. ZrO2-(15 wt%) HAP biocermic implant placed in a defect in the parietal bone of a rabbit. MSCT on the 3rd day after surgery.
To study tissue changes in the area of the bioceramic implant, MSCT of the rabbit skull was performed on days 7, 40, and 210 after surgery. The performed studies showed that during the entire observation period the implant was in the place of installation, there were no signs of pronounced dislocation. The bone structures also remained without signs of osteoporosis or osteolysis.
On the 7th day after the operation, soft tissue edema persisted, there were no signs of bone damage due to thermal exposure during the application of the burr hole. Densitometric bone density along the edge of the burr hole in contact with the implant was +223±31.5 HU. On the 40th day after the operation, there was no swelling of the soft tissues. In 11 animals, there was no displacement of the implant. In 3 rabbits, the deviation of the implant by 3-4 degrees from the original position was found. At the same time, there was a virtual disappearance of the gap between the implant and the bone. Densitometric bone density along the edge of the burr hole is +400±29.8 HU.
MSCT performed on the 210th day showed absolute preservation of the position of the implant, full contact between the bone tissue and the implant. The densitometric density of the bone tissue around the implant increased to +970±34.8 HU, which indicates a reliable fixation of the implant and its complete integration ith the bone (Figure 2).
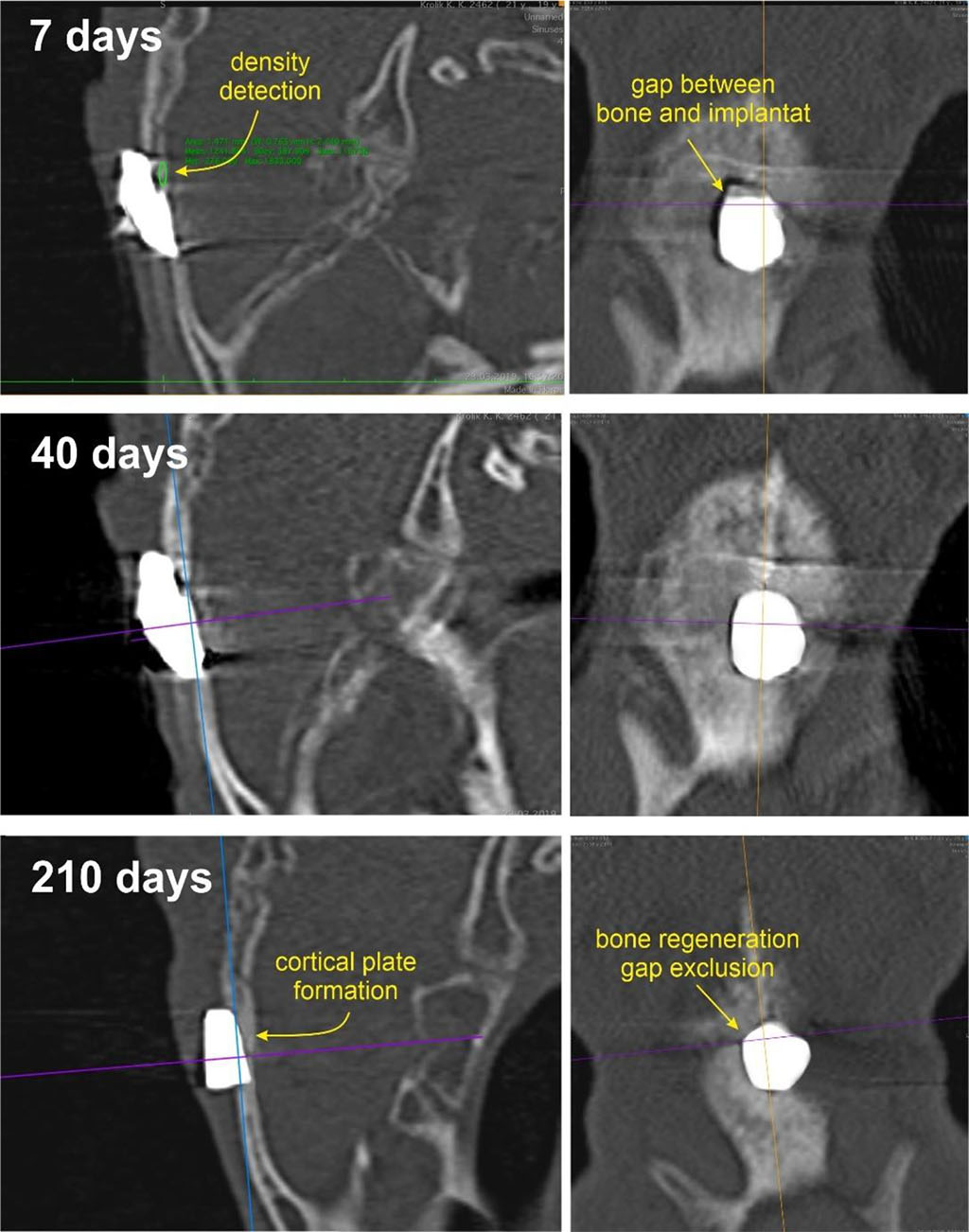
Figure 2. Computed tomography of the skull of a rabbit with a bioceramic implant in the defect zone on days 7, 40, and 210 after surgery.
In the control group, after 12-24 weeks, according to MSCT data, the bone defect persisted, which indicates the impossibility of its spontaneous healing. Surgical treatment of large bone defects is still a challenge for modern surgery. Their shelter often requires the implantation of a temporary or permanent prosthesis. The problem remains the creation of materials and products that meet the objectives of clinical practice. A biocompatible implant based on ZrO2 with a high specific surface area and good structural and mechanical properties was synthesized. The biological activity of the implant is provided by the presence of hydroxyapatite on its surface. In this study, a defect 10 mm in diameter was created in the parietal bone of rabbits. This minimal bone defect cannot be repaired and healed spontaneously, as shown in a study by Schlegel K.A. et al. (2006) [20]. A bioimplant based on cylindrical zirconium oxide was introduced into it.
The results of clinical observation in the postoperative period did not reveal infectious complications from the surgical wound, divergence of its edges above the implant zone, and visible signs of incompatibility. MSCT studies of the skull of rabbits were performed on days 7, 40, and 210 after surgery. These studies showed the absence of implant dislocation, its tight fixation in the bone defect, the absence of signs of osteoporosis and osteolysis in the surrounding bone tissue. Densitometric data revealed the formation of dense bone tissue in the implant area by 210 days after surgery.
In the group of animals in which the bone defect was not covered with an implant, after 12-24 weeks, no bone tissue was formed in the defect, which indicates that the area of the bone defect could not heal spontaneously.
The investigation was financially supported by Russian Science Foundation (project No. 18-73-10107). Link to information about the project: https://rscf.ru/project/18-73-10107/. Equipment of interdisciplinary CUC in the field of nanotechnologies and new functional materials (FEFU, Vladivostok, Russia) used in the research is gratefully acknowledged.