- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
The authors conducted a single-center clinical study of the effectiveness of endoscopic vacuum therapy for suture failure after bariatric surgery. The algorithm of actions was proposed, and the manipulations technique was described. Vacuum therapy was used in combination with a polyurethane obturator. The effectiveness of the technique was confirmed by positive results with the recovery of 8 patients. Endoscopic vacuum treatment of suture failure within the proposed algorithm is an effective method for treating the life-threatening complication of bariatric surgery.
Keywords: VAC therapy, anastomotic suture failure, bariatric surgery, endoscopic treatment, surgical complications.
Suture failure is a serious complication during operations on the hollow digestive tract organs, associated with a long inpatient treatment and fatal outcomes. In a series of early reports dating back to the 1990s, the incidence of esophageal anastomosis leakage after gastrectomy and proximal gastrectomy reached 30% [1–5]. Recent publications show a clear positive trend in the decline of the failure incidence, which is a reflection of an undoubted progress in surgical technique and instrumental refinement [6-8].
The choice of management tactics for each patient is individual. Endoscopic techniques have played a significant role in the diagnosis and treatment of this complication. Endoscopy allows diagnosing the presence of a defect, as well as assessing its location and size. In recent decades, a number of methods for endoscopic treatment of anastomoses and sutures of the digestive tract have been developed, which have a number of advantages over surgical and conservative management of this group of patients. Despite the accumulated experience, there is currently no unified systematic approach to the use of endoscopic methods for closing anastomotic defects [9,10].
The treatment strategy for a patient with incompetent sutures of the upper digestive tract simultaneously solves several problems: sanitation of the stagnation cavity and drainage of the mediastinum, providing nutritional support, closing the perforation, prevention and treatment of purulent complications. Lately, endoscopic vacuum therapy has been used to ensure adequate sanitation and drainage of the purulent cavity [11-13].
The purpose of this study was to evaluate the effectiveness of endoscopic vacuum therapy for suture failure in bariatric surgery and to develop a sequence of operations.
From 2020 to 2022, 289 bariatric surgeries were performed in the clinic, including 200 sleeve gastrectomies and 89 minigastric bypasses. Stapler anastomosis failure was diagnosed in 8 people 3-4 days after surgery. 3 patients underwent laparoscopic sanitation and drainage of the abdominal cavity, 5 patients underwent ultrasound-guided puncture of the leak, after which a vacuum aspiration system was adjusted for all patients.
Initially, it is necessary to ensure adequate sanitation and drainage of the purulent cavity, with the introduction of antiseptic solutions and agents. Usually this problem is solved surgically, depending on the nature of the previous intervention and the type of surgical access. In a number of cases, adequate sanitation can be achieved by ultrasound-guided or computed tomography-controlled drainage, and even using flexible endoscopes through the formed opening in the anastomosis. Fig. 1.
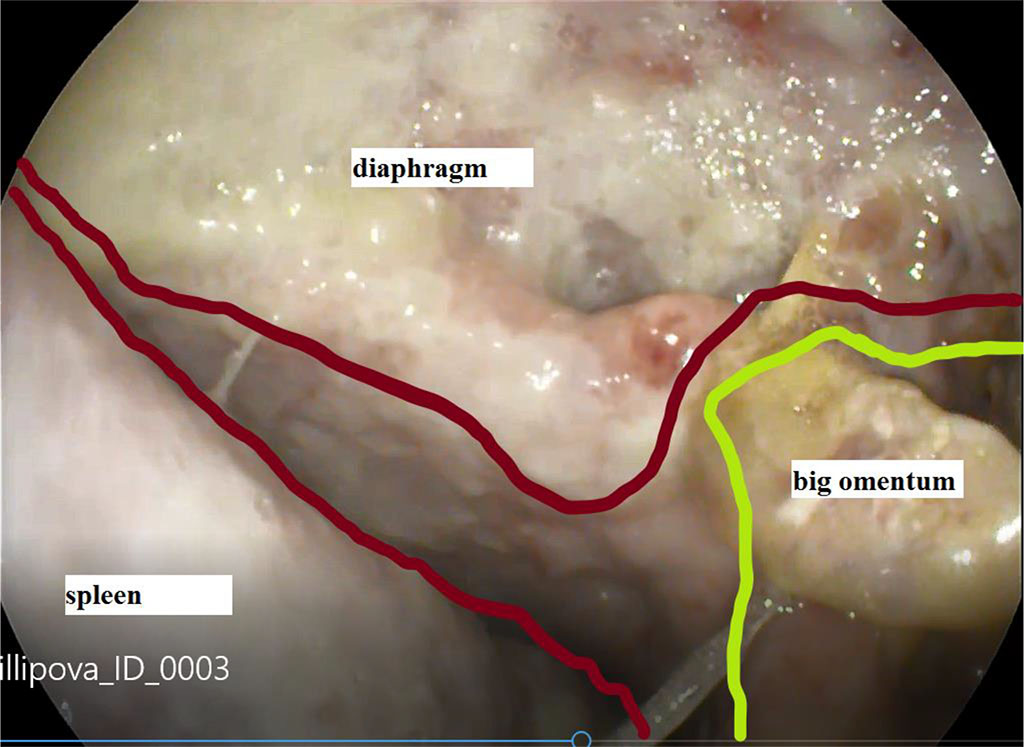
Figure 1. View of the abscess cavity of the left subdiaphragmatic space through a defect in the failure of gastric sutures
The operation was performed with the patient lying on his back. To ensure the stable respiratory function at all stages, orotracheal intubation was performed, followed by artificial lung ventilation. To reduce the risk of possible complications, the intervention was performed using carbon dioxide. During the initial study, the level of location of the anastomotic leak was noted, measuring it in centimeters from the incisors. The size of the leak and the local state of the tissues were assessed, as well as the degree and severity of inflammatory and regenerative changes. When technically possible and the patient's condition was stable, we inspected the leak cavity in the mediastinum or abdominal cavity, connected with the site of the leak, and the fistulous tract. We assessed their sizes, and the state of the surrounding tissues and determined the severity of inflammatory changes, as well as the presence of sequesters. If necessary, the cavity was counterstained using a water-soluble contrast to detail the revealed changes, assess the configuration of the cavity, diagnose latent leaks and determine the adequacy of the drainage function. Primary sanitation of the cavity with the removal of fibrin, saliva and intestinal contents and necrotic tissues was carried out by abundant rinsing with sterile solutions with delicate aspiration of the contents, excluding damage to adjacent anatomical structures and, above all, blood vessels. Also, during the initial study, we determined the technical feasibility of inserting a porous polyurethane system into the cavity lumen without the risk of additional anastomotic injury.
Subsequently, the endoscope was passed below the level of the anastomosis at a distance of at least 50 cm with the installation of a nasoenteric nutritional tube.
In accordance with the information about the size of the leak and the lumen of the esophagus, the final decision was made on the placement of the porous system - inside the esophagus or inside the cavity. Depending on the decision made, the primary sponge system was cut out from the polyurethane sponge plate using Cooper's scissors and was modeled to a cylindrical shape. When the sponge was located intraesophageally, its average diameter corresponded to the diameter of the esophagus at the level of the anastomosis or was slightly smaller than the latter. When located inside the cavity, the diameter of the spongy cylinder corresponded to the size of the defect, but was not larger than the diameter of the esophagus. The length of the intraesophageal spongy implant was determined by the length of the defect with overlapping of the wound edges by 3 cm on both sides and most often was 6-8 cm or larger.
The porous sponge, mounted on a gastric tube, was located parallel to the endoscope and, immediately before insertion, was grasped by the right hand along with the inserted part of the apparatus as a single system. The subsequent insertion of the system was carried out under visual control through the endoscope. To reduce the effort during the system insertion and to overcome the natural resistance in the area of anatomical narrowing, it is advisable to use a rigid endoscope, moisten the sponge with water or a water-soluble gel, slightly unbend the head in the cervical region in order to straighten the bend in the pharyngeal-esophageal region.
The level of location of the porous system was determined under direct visual control through the endoscope. Given that the sponge is located outside the field of vision of the apparatus, the latter was passed more distally from the defect level into the anastomosed intestine or stomach to a depth of 4–5 cm. Subsequently, the clip inserted through the endoscope channel and fixing the porous system at the distal end by the loop was released and taken out. The sponge was positioned under visual control through the endoscope at the required level. Contrasting the sponge and the fistulous tract with a water-soluble contrast through a gastric tube allowed for additional X-ray control and determining the location of the system relative to the perforation level. Immediately after installation, the tube was fixed to the nasal septum or concha and connected to active suction with a vacuum level of 100-110 mm Hg.
The suction system replacing was performed after a preliminary esophagoscopy. At the same time, tissues were examined in the area of the anastomosis and adjacent areas of the esophagus and stomach. In the presence of granulations and regenerative changes, we tried to conduct a circular revision with careful detachment of the sponge from the adjacent tissues and the release of granulations from the porous system under visual control. Subsequently, this made it possible to reduce resistance when removing the sponge and reduce tissue bleeding in case of granulation injury. The sponge was pulled up to the level of the oral cavity, grasped with a Mikulich forceps under visual control, and removed from the oral cavity. The gastric tube was cut with scissors and removed from the nasal cavity. During the control endoscopy, we assessed regenerative changes in tissues, the size of the defect, the condition of the tissues in the adjacent cavity if it was available for endoscopic revision. If necessary, the position of the drains was corrected and they were pulled up if they protruded into the lumen of the digestive tract through an anastomotic defect. The subsequent installation of the aspiration system was carried out according to the previously described method.
In all cases of suture failure, the use of the above methodology was successful. After 7 days of endoscopic vacuum therapy, we noted a decrease in the size of the defect by more than 2 times. Fig. 2, 3. Seven patients were discharged from the hospital 14±2 days after surgery without any complications. One patient was discharged on the day 17 due to the formed abscess communicating with the cavity of the stomach stump. For this patient, sanitation continued once every two days on an outpatient basis. She recovered on the day 25.
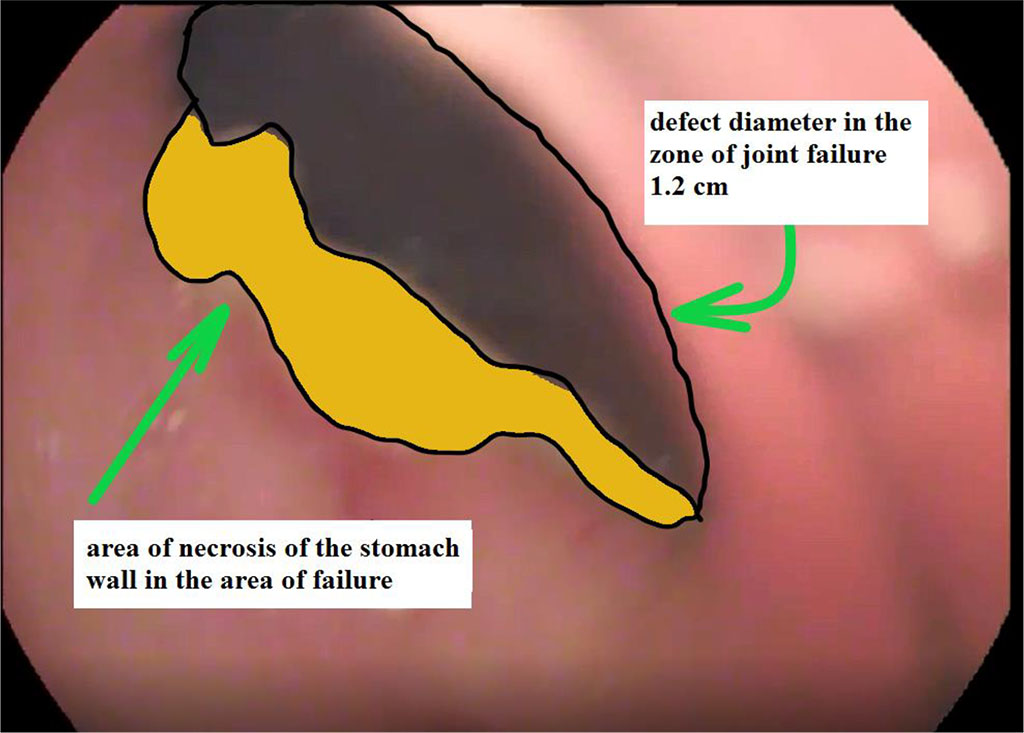
Figure 2. View of a gastric suture defect with a zone of near-wound necrosis at the time of detection
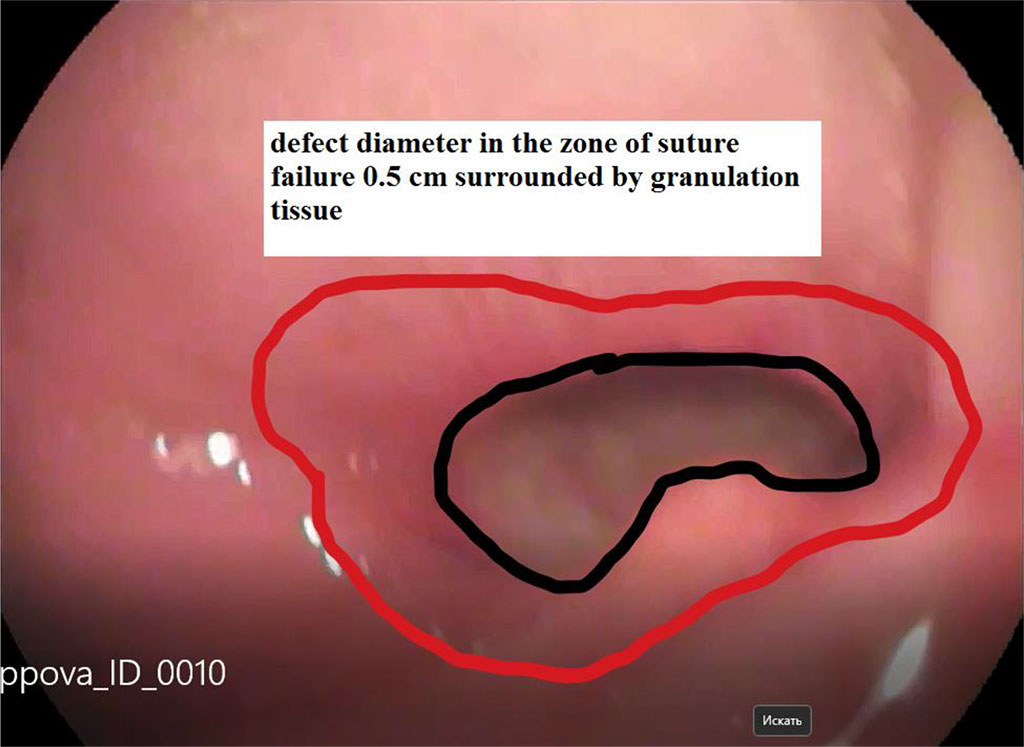
Figure 3. View of the defect 7 days after the start of the vacuum endoscopic treatment
Given that this study was conducted in just one medical center, further research is required to improve the methodology.
The use of endoscopic vacuum treatment for suture failure within the proposed algorithm is an effective method for treating this type of complications after bariatric surgery.