- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Premature ventricular contractions (PVCs) are not included in the risk factors for arterial thromboembolism.
Aim: To determine the relationship between PVCs and the development of arterial thromboembolic complications.
Methods: The study included 440 patients with PVCs 700 or more per 24 hours, control group - 88 people with PVCs less than 700. All patients underwent laboratory and instrumental studies: lipid spectrum, hemostasis indicators; 24-hours ECG monitoring; echocardiography (EchoCG); Doppler ultrasound and digital sphygmography (SG) of the main arteries; ultrasound of the aorta branches, renal arteries. According to the indications - stress EchoCG with physical exercises, coronary angiography, pancerebral angiography, renal arteries angiography, computed tomography, magnetic resonance imaging of the brain. All patients of the main group were divided into 2 subgroups, depending on the moment of occurrence of PVCs ventricular systole in the cardiac cycle, regardless of the ectopic center: subgroup A (120) - patients with PVCs before the peak of transmitral blood flow; subgroup B (320) - after the peak of the transmitral blood flow. Patients were observed during 1 year and the development of arterial thromboembolic events was analyzed.
Results: According to the main clinical, laboratory and instrumental data, patients of subgroups A, B and the control group were equivalent. Also, during 1 year, a statistically significantly higher development of arterial thromboembolic events was observed in subgroup A. When comparing linear blood flow velocity and volumetric blood flow, there was a significant increase in parameters during the spreading of the first post-extrasystolic contraction wave. A similar trend was observed in the parameters of the kinetics of the arterial vascular wall (velocity, acceleration, power, work).
Conclusion: PVCs are an additional risk factor for arterial thromboembolic events. The main danger is not the PVC itself, but the wave of the first post-extrasystolic contraction, which can become the starting point for the instability of atherosclerotic plaques, leading to tears, parietal thrombosis, and embolism in the arterial vessels.
Keywords: arterial thromboembolic events; arterial hemodynamics; premature ventricular contractions
Premature ventricular contractions (PVCs) are the most frequent type of heart arrhythmias in humans, they are observed approximately in 80-95% of the population [1-3]. Moreover, if PVCs are monotopic, rare, then usually cardiologists do not treat them by antiarrhythmic drugs. The existing classifications of PVCs are based, most often, on electrophysiological principles, and do not take into account at what moment in the cardiac cycle the ventricular systole of PVCs occurs [4-7]. Thus, hemodynamic changes occurring in the heart and the main arterial vessels are not taken into account. We wrote about the objectivity of using this functional classification earlier in our works [8].
At the same time, the incidence of arterial thromboembolic events remains high, despite of preventive measures. At the same time, among arrhythmias, atrial fibrillation is within the risk factors for arterial thromboembolic complications. It is a potentially dangerous arrhythmia, leading to the formation of intracardiac thrombi (left atrial appendage or apical thrombosis of the left ventricular apex in the case of an aneurysm), which can become a source of cardioembolic events. To prevent them, anticoagulants are prescribed, and although against the background of their use it is possible to reduce the likelihood of cardiocerebral events, the risks associated with their use do not always outweigh the benefits, and complications occur [9-13].
According to the American Stroke Association, up to 45% of registered ischemic strokes are classified as cryptogenic. However, in these patients all traditional risk factors for the development of stroke were excluded. A number of foreign studies described a higher incidence of strokes in patients with frequent PVCs [14–19]. However, until now, PVCs has not been included in the list of risk factors for arterial thromboembolic events. These contradictions and inconsistencies caused our interest in performing this work.
Purpose of the study: To determine the relationship between PVCs and the development of arterial thromboembolic complications.
The study included 440 (318 men and 122 women) patients of clinics of Samara State Medical University, Samara, Russia. Inclusion criteria: PVCs 700 or more per day; signed agreement for participation in the study. Exclusion criteria: heart aneurysms; aortic aneurysms; atrial fibrillation; intracardiac thrombosis; heart myxomas; dilated cardiomyopathy; prosthetic heart valves; chronic heart failure (HF) more severe than NYHA II; diabetes mellitus; chronic obstructive pulmonary disease more severe than mild severity; hematological diseases; smoking experience of 7 years or more; hemodynamically significant stenosis of the carotid bifurcation (≥70%). In the control group, we included 88 patients with PVCs less than 700 per day. Patients with ventricular and supraventricular tachycardias, sinus node weakness, implanted pacemaker were not included in the study. Thus, at the stage of patient selection, the effects of traditional risk factors for arterial thromboembolic events were excluded or minimized.
All patients underwent laboratory and instrumental research methods: lipids levels (cholesterol, low density lipoproteins, high density lipoproteins, triglycerides), hemostasiograms; 24-hours ECG monitoring; transthoracic or transesophageal echocardiography (EchoCG); Doppler ultrasound and digital sphygmography (SG) of the different types of arteries (a. carotis communis, a. tibialis posterior); Doppler ultrasound of the aorta branches, renal arteries. According to the indications we performed stress EchoCG with physical exercises, coronary angiography, pancerebral angiography, angiography of the renal arteries, computed tomography or magnetic resonance imaging of the brain. With Doppler ultrasound, the main parameters of arterial hemodynamics (linear blood flow velocity (LBV), volumetric blood flow) were determined. In digital SG, we detected the main parameters of the arterial vascular wall kinetics (velocity, acceleration, power, work), measured during the spread of regular, extrasystolic and first post-extrasystolic pulse waves. Carotid arteries atherosclerosis was diagnosed using Doppler ultrasound methods. The characteristics of the plaques (echogenicity, heterogeneity, the presence of calcium inclusions, the nature of the surface) were assessed in the gray scale mode, the percentage of stenosis by diameter (on the EST scale), and the speed characteristics before and at the site of maximum stenosis were assessed using the NASCET, St .Mary's ratio criteria. So, it was possible to most fully study intra-arterial hemodynamic processes, as well as the effect of PVCs on changes in the main analyzed parameters.
Based on the features of the biomechanics of the identified PVCs, all patients of the main group were divided into 2 subgroups depending on the moment of occurrence of ventricular systole of PVCs in the cardiac cycle, regardless of the ectopic center: subgroup A (120 people) - patients with PVCs that occurred before the peak of transmitral blood flow (peak E on EchoCG); subgroup B (320 people) - with PVCs after the peak of the transmitral blood flow. So, both supraventricular and ventricular PVCs could occur in different phases of the cardiac cycle: before or after the peak of the transmitral blood flow. The peak of the transmitral blood flow was determined according to the data of an echocardiographic study: the E peak was analyzed. All patients initially underwent investigations on the background of therapy prescribed on an outpatient stage. After, if necessary, the treatment was corrected in correspondence to the current recommendations of the European and Russian Societies of Cardiology.
Patients were followed up for 1 year from the onset of the study. During the observation period, analysis of the development of arterial thromboembolic events (stroke or transient ischemic attack, myocardial infarction, distal arterial embolism of the arteries of the lower extremities) was performed by questioning or, in case of readmission to the hospital, by examining the data from the case histories. The additional visits were at the 6 and 12 months from the onset of the study.
Our study had a number of limitations. So, during the observation period of 1 year, it was impossible to completely exclude the development of PVCs of other gradations. In this sense, this task technically presented certain difficulties, since even multiple repetitions of 24-hours ECG monitoring do not exclude such a possibility. However, in our clinical practice, we observed the following pattern: when antiarrhythmic therapy was stopped, most often PVCs of the same grade recurred. In addition, despite the limitations described above, a sufficient statistical sample was used in the study (440 patients in the main group + 88 people in the control group), which allowed us to obtain reliable results. Secondly, in our work, we focused on studying the features of hemodynamics and kinetics during the passage of the pulse wave of the first post-extrasystolic contraction in the arteries, showing its potential danger. Moreover, our clinical findings were subsequently confirmed by the experimental part of the work using the "Device for the modeling of intra-arterial circulation", which is described in detail in a previously published article [19].
The analysis of statistical data was based on the principles of evidence-based medicine. A one-way analysis of variance (ANOVA) was performed to compare mean values of independent groups for each analyzed parameter. Parameters at which p≤0.05 were considered statistically significant. The main parameters of hemodynamics (LBF, volumetric blood flow) were analyzed according to ultrasound data and the kinetics of the main arteries (velocity, acceleration, power, work) according to digital SG. Cox analysis was used to assess the development of arterial thromboembolic events within 1 year. In addition, indicators of a four-field table were calculated to establish the relationship between the development of arterial thromboembolic events within 1 year and the presence of PVCs before the peak of transmitral blood flow in the cardiocycle.
According to most of the main clinical, laboratory and instrumental data, patients of subgroups A, B and the control group were identical (table 1).
Table 1. Clinical, laboratory and instrumental data of subgroups A, B and the control group.
| Parameter | Subgroup А n=120 | Subgroup B n=320 | Control group n=88 | p |
| Mean age, М±σ, years old | 63,1±5,3 | 62,8±6,1 | 61,9±5,9 | p>0.05 |
| Women – n (%) | 24 (20) | 98 (31) | 43 (49) | p (А, B)=0.1534, p (А, c)=0.0001, p (B, c)=0.0154 |
| Men – n (%) | 96 (80) | 222 (69) | 45 (51) | |
| Arterial hypertension 1 degree – n (%) | 43 (36) | 128 (40) | 37 (42) | p (А, B)=0.9315, p (А, c)=0.8941, p (B, c)=0.9969 |
| Arterial hypertension 2 degree – n (%) | 74 (62) | 192 (60) | 45 (51) | p (А, B)=0.9978, p (А, c)=0.5545, p (B, c)=0.5688 |
| HF NYHA I – n (%) | 30 (25) | 83 (26) | 25 (28) | p (А, B)= 0.9996, p (А, c)= 0.9821, p (B, c)=0.9905 |
| HF NYHA II – n (%) | 90 (75) | 237 (74) | 59 (67) | p (А, B)=0.9996, p (А, c)=0.7194, p (B, c)=0.8272 |
| Myocardial infarction in anamnesis – n (%) | 24 (20) | 67 (21) | 17 (19) | p (А, B)=0.9996, p (А, c)=0.9821, p (B, c)=0.9819 |
| Stroke or transient ischemic attack in anamnesis – n (%) | 13 (11) | 34 (11) | 6 (7) | p (А, B)=1.0000, p (А, c)=0.8598, p (B, c)=0.8261 |
| Arterial thromboembolic complications of the lower extremities – n (%) | 1 (1) | 3 (1) | 1 (1) | p (А, B)=1.0000, p (А, c)=0.9995, p (B, c)=0.9998 |
| Absence of intra-heart thrombi – n (%) | 120 (100) | 320 (100) | 88 (100) | p=1.0000 |
| Ejection fraction by Simpson ≥50% – n (%) | 2 (2) | 18 (6) | 0 (0) | p (А, В)=0.3894, p (А, c)=0.7434, p (В, c)=0.1539 |
| Ejection fraction by Simpson 40-49% – n (%) | 108 (90) | 289 (90) | 88 (100) | p (А, В)=1.0000, p (А, c)=0.0195, p (В, c)=0.0205 |
| Ejection fraction by Simpson <40, % – n (%) | 10 (8) | 13 (4) | 0 (0) | p (А, B)=0.0244, p (А, c)=0.0446, p (В, c)=0.6292 |
| Hemodynamically insignificant carotid bifurcation stenosis – n (%) | 42 (35) | 66 (21) | 5 (6) | p (А, B)=0.0157, p (А, c)<0.0001, p (В, c)=0.0095 |
| Type IIIB atherosclerotic plaque in carotid bifurcation– n (%) | 20 (17) | 21 (7) | 1 (1) | p (А, В)=0.0104, p (А, c)=0.0023, p (В, c)=0.2693 |
| Insignificant coronary arteries stenosis – n (%) | 96 (80) | 263 (82) | 68 (77) | p (А, В)=0.9847, p (А, c)=0.9897, p (В, c)=0.8358 |
| Cholesterol ≥5,0 mmol/l – n (%) | 35 (29) | 101 (32) | 24 (27) | p>0.05 |
| LDL-cholesterol ≥4 mmol/l – n (%) | 6 (5) | 13 (4) | 4 (5) | p>0.05 |
| HDL-cholesterol<1 mmol/l – n (%) | 8 (7) | 24 (7) | 3 (3) | p>0.05 |
Despite the comparability of the initial parameters of the subgroups, within 1 year from the onset of the study, a statistically significantly higher incidence of arterial thromboembolic events was observed in subgroup A (Table 2).
Table 2. Arterial thromboembolic complications in 1 year from the onset of investigation.
| Arterial thromboembolic complications | Subgroup A* n=120 | Subgroup В* n=320 | Cонтрольная группа* n=88 |
| Stroke or transient ischemic attack – n (%) | 9 (7,5) | 11 (3,4) | 0 (0) |
| Myocardial infarction – n (%) | 6 (5,0) | 8 (2,5) | 1 (1,1) |
| Arterial thromboembolic complications of the lower extremities – n (%) | 2 (1,7) | 1 (0,3) | 0 (0) |
| Summary – n (%) | 17 (14,2) | 20 (6,2) | 1 (1,1) |
*p (A, В)=0.0080, p (A, c)<0.001, p (В, c)=0.0458
When calculating the indicators of the four-field table, the normalized value of the Pearson coefficient (C') was 0.316, which indicated the average strength of the relationship between the presence of PVCs flow more than 700 per 24 hours with ventricular systole before the peak of transmitral blood flow and the development of thromboembolic events.
Thus, at PVCs more than 700 per day, there was a more frequent development of arterial thromboembolic events, especially in PVCs with ventricular systole before the peak of transmitral blood flow in the cardiac cycle. How we could explain these results? In order to answer this question, we analyzed the main parameters of hemodynamics and kinetics of the arteries and compared them in selected subgroups. So, when comparing LBF and volumetric blood flow according to ultrasound data, there was a significant increase in these parameters during the passage of the wave of the first post-extrasystolic contraction both in the arteries of the elastic type (a. carotis communis) and in the arteries of the muscular-elastic type (a. tibialis posterior) (table 3).
Table 3. LBF, volume blood flow in the first post-extrasystolic wave in subgroups A, B and control group.
| Doppler ultrasound parameter | Subgroup А n=120 | Subgroup В n=320 | Control group n=88 | |
| LBF, M±σ, m/s | a. carotis communis | 1,21±0,36* | 0,94±0,22* | 0,72±0,26* |
| a. tibialis posterior | 0,49±0,18* | 0,36±0,14* | 0,29±0,16* | |
| Volume blood flow, M±σ, ml/min | a. carotis communis | 576±54* | 432±39* | 360±48* |
| a. tibialis posterior | 59±23* | 48±21* | 36±17* | |
*p (А, В), р (А, c), р (В, c) <0.05
A similar tendency was also observed in the analysis of the parameters of the kinetics of the arterial vascular wall (velocity, acceleration, power, work). Figures 1, 2 show the summary change in the power parameter by a. carotis communis, a. tibialis posterior calculated in the phases of inflow over outflow and outflow over inflow.
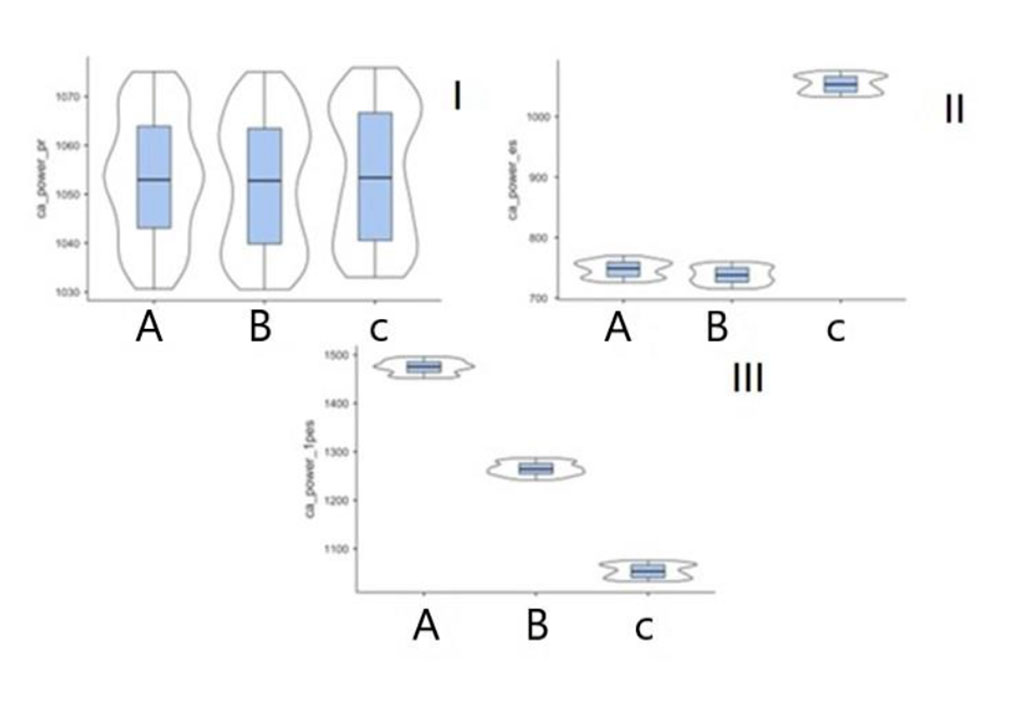
Figure 1. Power parameter (р<0.001) in patients of subgroups A, B and control group in digital SG on a. carotis communis (I – pre-extrasystolic contraction, II – premature ventricular contraction, III – first post-extrasystolic contraction).
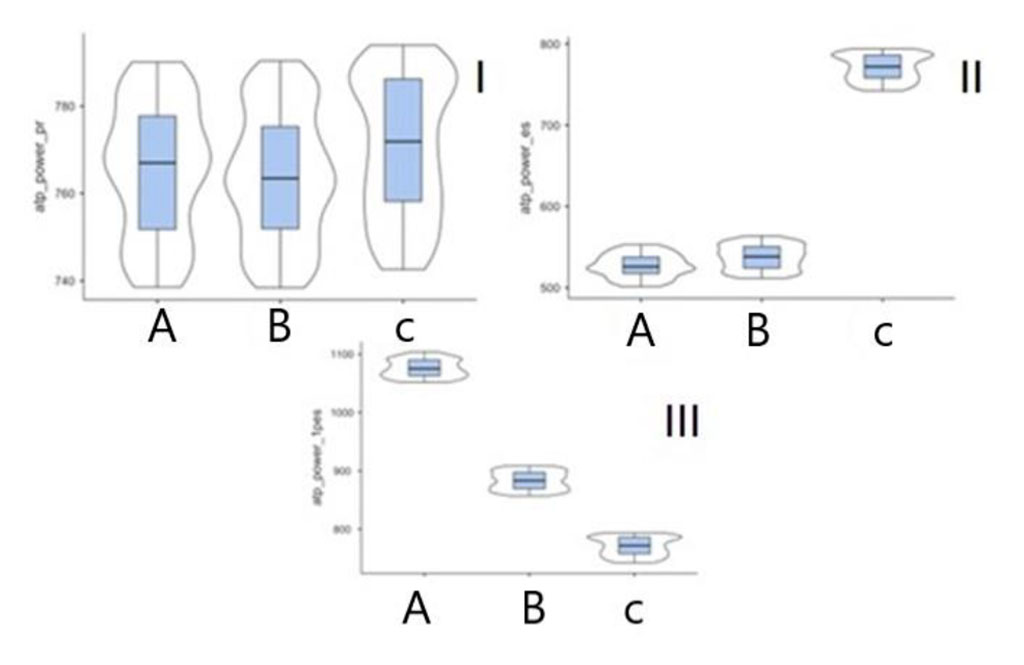
Figure 2. Power parameter (р<0.001) in patients of subgroups A, B and control group in digital SG on a. tibialis posterior (I – pre-extrasystolic contraction, II – premature ventricular contraction, III – first post-extrasystolic contraction).
Scientific literature describes the more frequent development of cryptogenic stroke in patients with PVCs [1, 2]. However, there is still no explanation of this fact. Early studies have reported worsening of cerebral circulation with frequent PVCs [20]. A decrease in the mean blood flow velocity in the middle cerebral artery during ventricular PVCs has also been demonstrated [21]. A number of researchers have associated the development of cryptogenic strokes in PVCs with more frequent development of atrial fibrillation in these patients. Thus, the ASSERT and CRYSTAL AF studies showed that one of the possible causes of cryptogenic stroke is cerebral embolism in non-diagnosed atrial fibrillation [21, 22]. According to the current ESUS terminology of cryptogenic stroke, atrial fibrillation, leading to the formation of cardioembolic sources, is described among the possible causes among rhythm disturbances [23]. Thus, PVCs are still not included in any of the scales for assessing the risk of thromboembolic complications.
We believe that we should pay attention to the higher incidence of arterial thromboembolic events during 1 year of observation in patients with PVCs more than 700 per day. At the same time, traditional risk factors for thromboembolic complications were minimized at the stage of patients selection for the investigation. The subgroups were identical in the main clinical, laboratory and instrumental research methods. The main difference between subgroups A and B and the control group was in the revealed patterns of hemodynamic and kinetic parameters. The following tendency was observed: during the passage of the wave of the first post-extrasystolic contraction, an increase in the main parameters of hemodynamics (LBF, volumetric blood flow) and kinetics (velocity, acceleration, power, work) was observed more in subgroup A, in comparison with subgroup B and the control group. In other words, the earlier the ventricular systole of PVCs occurred in the cardiac cycle, the more significant increase in the analyzed parameters was observed. The moment of occurrence of the ventricles’ systole of the PVCs predetermined the hemodynamic changes in the intra-arterial circulation. For the first time, this pattern was described by us in a previously published work [8].
When performing Doppler ultrasound of the arteries, the doctor, determining the parameters of LBF, volumetric blood flow, usually focuses on the data measured at the regular heart rhythm. If there’s PVC, such measurements are usually not included in the final protocol. In our study, we calculated hemodynamic parameters during the regular heart rhythm wave, as well as during the PVCs wave and first post-extrasystolic contraction.
Some patients of both subgroups A and B of the main group had atherosclerotic lesions of the carotid and coronary arteries. At the same time, there were no indications for surgical correction in these patients, since because the stenoses were hemodynamically insignificant. However, among the identified atherosclerotic plaques, there were those that had an uneven surface, were calcified, or had other signs characterizing a potentially dangerous plaque, which under certain conditions could become a source of arterial embolism. We reasonably believe that the wave of the first post-extrasystolic contraction could become the starting point in the development of arterial thromboembolic events in this category of patients. At the same time, the greatest danger from this point of view is not the PVC itself, but the wave of the first post-extrasystolic contraction, especially in PVCs, the ventricular systole of which occurs in the early phase of the cardiac cycle - before the peak of the transmitral blood flow.
The clinical patterns obtained in this work were also confirmed by an experimental study using the “Device for modeling of intra-arterial circulation” created by us [19]. This useful model allowed us, by simulating the propagation of pulse waves during PVCs in an arterial vessel, to visualize and quantify hemodynamic parameters in various PVCs variants. Thus, with a piezocrystalline sensor, during the spread of the wave of the first post-extrasystolic contraction during PVCs to the peak of the transmitral blood flow, an increase in pressure inside the device was recorded up to a maximum of 1?6 times compared with the pressure at the regular heart rhythm.
PVCs should be considered as an additional risk factor for arterial thromboembolic events. The greatest danger is not the PVCs itself, but the wave of the first post-extrasystolic contraction, which can become the starting point for the instability of atherosclerotic plaques, leading to their tears, parietal thrombosis, and arterial embolism.
Nothing to declare