- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 5. DOI 10.35630/2025/15/Iss.5.508
Background: Frailty is a progressive geriatric syndrome characterized by decreased physiological reserve, reduced resilience to stressors, and increased risk of falls, disability, hospitalization, and mortality. Recent research has reframed frailty as a dynamic and potentially reversible condition. Physical activity (PA) is one of the most promising non-pharmacological strategies for its prevention and management.
Aims: To summarize and critically evaluate current evidence on the role of physical activity in the development, prevention, and reversal of frailty in older adults, based on recent systematic and narrative reviews published between 2019 and 2024.
Methods: This narrative evidence-based review synthesized data from systematic reviews and meta-analyses retrieved from PubMed, Scopus, Embase, Web of Science, and Cochrane CENTRAL (2019–2024). Studies including adults aged ≥60 years were analyzed, focusing on aerobic, resistance, balance, multicomponent, and mind–body exercise interventions. Outcomes included frailty incidence, functional capacity, cognition, and quality of life. Methodological rigor was assessed according to PRISMA and GRADE criteria reported by the original authors.
Results: Higher levels of physical activity were consistently associated with an approximately 40–55% lower risk of developing frailty. Multicomponent exercise programs combining resistance, aerobic, and balance training produced the strongest and most consistent improvements in frailty status, gait speed, muscle strength, and cognitive performance. Biological mechanisms underlying these benefits include mitigation of sarcopenia, reduction of chronic inflammation and oxidative stress, improvement of mitochondrial efficiency, and regulation of anabolic hormones.
Conclusions: Regular, structured, and individualized physical activity represents an evidence-based therapeutic strategy for preventing and reversing frailty in older adults. Integration of tailored multicomponent exercise programs into clinical and community care should be prioritized. Future research should focus on standardizing frailty and activity assessment, defining optimal dose–response relationships, and expanding evidence to diverse populations.
Keywords: physical activity, exercise, frailty, older adults, geriatric syndrome, narrative review, meta-analysis
The rapid global aging of populations has transformed the structure of modern societies and created new medical and socioeconomic challenges. One of the most pressing among them is frailty, a complex geriatric syndrome that represents a state of increased vulnerability to internal and external stressors due to progressive decline in physiological reserves. Frailty leads to a marked rise in morbidity, disability, hospitalization, institutionalization, and premature mortality, exerting a significant burden on healthcare systems worldwide [1,2]
Although frailty was traditionally viewed as an unavoidable consequence of aging, contemporary research has reframed it as a dynamic and potentially reversible clinical condition. This paradigm shift has redirected attention from passive observation of decline to active prevention and intervention strategies [2]. The understanding that frailty can be modified through targeted measures has opened new opportunities for improving the quality of life and independence of older adults.
Among non-pharmacological approaches, physical activity occupies a central role. It contributes to maintaining muscle mass, strength, balance, metabolic homeostasis, and cognitive function. Recent studies indicate that structured and individualized exercise programs can prevent or even reverse frailty manifestations, reduce inflammation and oxidative stress, and improve mitochondrial function [3–6]. These findings confirm that physical activity represents an effective and evidence-based approach for managing frailty and promoting healthy aging.
The relevance of this topic is determined by the growing prevalence of frailty in aging populations and the urgent need for practical, non-pharmacological strategies that can be implemented in routine clinical care. The novelty of this review lies in the integration and critical analysis of recent evidence from systematic and narrative reviews published between 2019 and 2024, focusing on the biological mechanisms of adaptation to exercise, the comparative efficacy of different modalities, and their applicability across frailty phenotypes [4–7].
The aim of this narrative evidence-based review is to summarize and critically assess the latest findings on the role of physical activity in the development, prevention, and management of frailty. The review discusses the conceptual models of frailty, current exercise recommendations for older adults, evidence supporting various types of physical activity, underlying biological mechanisms, and emerging insights into optimal dose–response relationships [3–7].
This narrative evidence-based review synthesizes findings from systematic reviews and meta analyses to provide an up-to-date summary of the relationship between physical activity (PA) and frailty in older adults. This approach ensures that the conclusions drawn are based on the highest level of aggregated evidence available.
The electronic databases searched included PubMed, Scopus, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL). The search covered the period from January 2019 to April 2024. The search strategy combined the following keywords and their variations: “physical activity,” “exercise,” “frailty,” “older adults,” “geriatric syndrome,” “systematic review,” “meta-analysis.”
Inclusion criteria: systematic reviews and meta-analyses of randomized controlled trials (RCTs) or longitudinal cohort studies involving community-dwelling or institutionalized adults aged 60 years or older. Only studies written in English and providing quantitative or qualitative data on frailty status, physical and cognitive function, or quality of life were included.
Exclusion criteria: publications without clear methodological transparency, studies not focused on physical activity or frailty, narrative summaries without systematic data extraction, and duplicate or low-quality reviews. Study selection was conducted independently by two reviewers who screened titles, abstracts, and full texts. Disagreements were resolved by discussion and consensus.
Data extraction included study design, sample size, participant characteristics, type, duration, and intensity of physical activity, frailty assessment tools, main outcomes, and limitations reported by each review.The methodological quality of the included reviews was evaluated using the criteria reported by theoriginal authors, including adherence to PRISMA guidelines, PROSPERO registration, and risk- of-bias tools such as GRADE, Cochrane RoB, or the Newcastle–Ottawa Scale.
Data synthesis was descriptive and comparative. Results were grouped according to the type of physical activity (aerobic, resistance, balance, multicomponent, or mind–body training) and analyzed with attention to consistency of outcomes, reported effect sizes, and methodological rigor. This methodology aims to ensure transparency, reproducibility, and a comprehensive overview of the evidence base, consistent with current standards for narrative evidence-based reviews in biomedical research.
The following diagram illustrates the general process of literature identification and selection
for this narrative evidence-based review. The numbers represent the overall volume of studies
screened and included based on the criteria described in the Methods section.
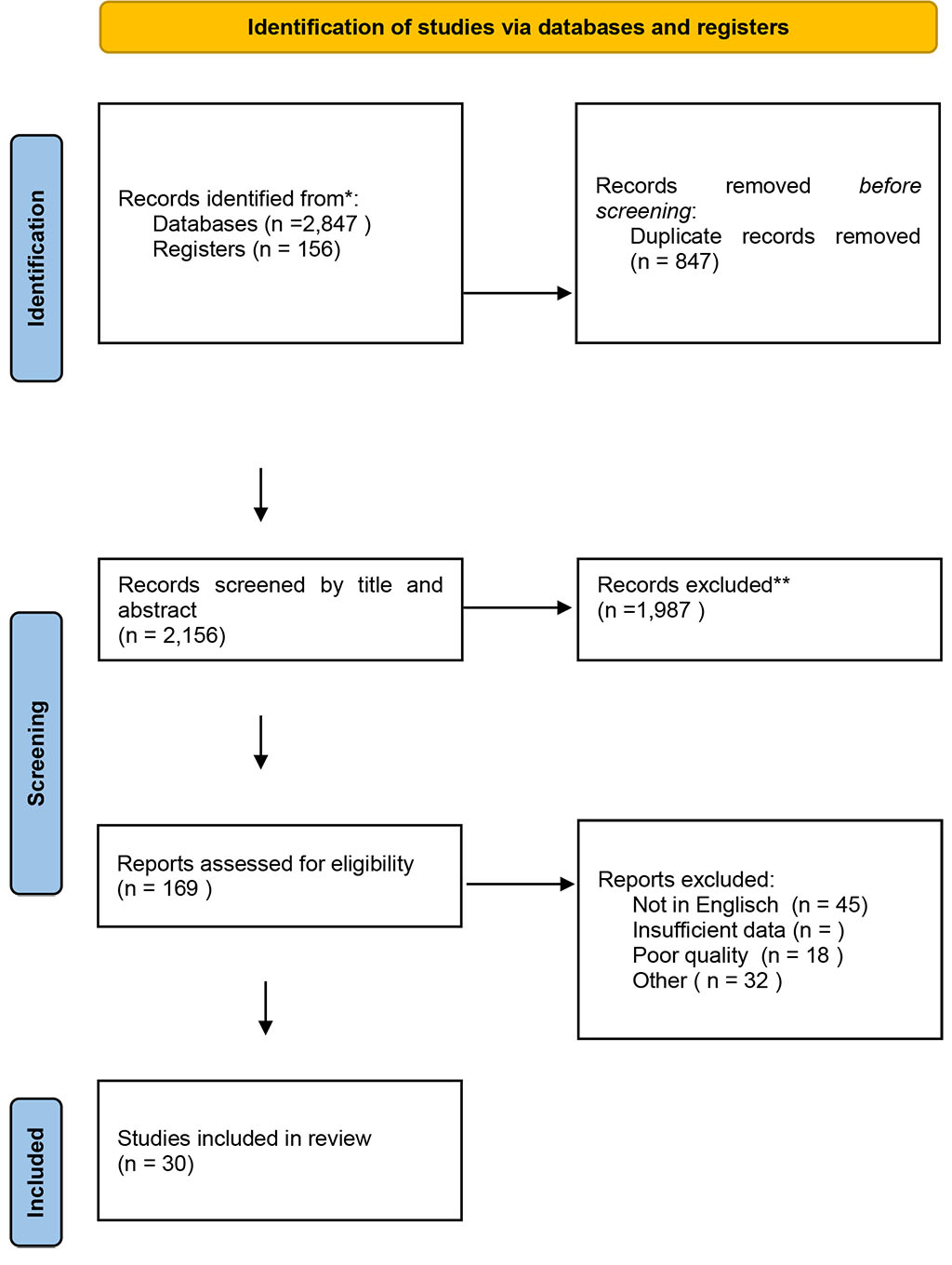
Figure 1. PRISMA flow diagram of study selection
Table 1. Characteristics of included systematic reviews and meta-analyses (2019–2025)
| Author (Year) | Study Type | Population / Sample Size | Intervention Type | Duration / Frequency | Main Outcomes | Key Findings | Limitations |
| Zhao et al., 2022 [3] | Systematic review and meta-analysis of cohort studies | 28 studies; >60,000 older adults | Total physical activity (various types) | 6–60 months | Frailty incidence | Higher PA associated with 41% lower odds of frailty | Heterogeneity of PA definitions |
| Caicedo-Pareja et al. (2024)[8] | Systematic review & meta-analysis | 15 studies, frail/pre-frail | Multicomponent exercise, | 8–24 weeks | Frailty status, physical performance | Improvements in strength, balance, gait speed; frailty reversal | Variable intensity/adherence |
| Lim et al., 2024 [5] | Systematic review and meta-analysis of RCTs | Pre-frail adults (n=2,300) | Community-based multicomponent exercise | 12–48 weeks; 2–3×/week | Physical function, frailty status | Significant improvements in function and QoL | Small sample sizes in several studies |
| Sirikul et al. (2024) [9] | Meta-analysis of RCTs | 18 RCTs, 3,457 older adults | Multicomponent exercise ± nutrition | 8–52 weeks | Frailty incidence | PA reduced frailty risk by 55% (95% CI 45–67%) | Heterogeneity of programs |
| Wang et al., 2024 [6] | Systematic review and meta-analysis of RCTs | Frail elderly (n=1,950) | Multicomponent exercise (strength, balance, endurance) | 8–52 weeks | Frailty status, SPPB, TUG | Significant gains in strength, gait speed, balance | Short intervention periods |
| Martínez-Montas et al. (2025) [10] | Systematic review & meta-analysis of RCTs | 1,223 institutionalized older adults (12 RCTs) | Multicomponent exercise | 8–24 weeks | Frailty reversal, mobility | 36% frailty reversal; greater effects with cognitive/nutritional add-ons | Limited generalizability (institutional setting) |
| Mello et al., 2022 [7] | Review of clinical trials | Frail adults aged ≥65 | Multicomponent and resistance training | Variable | Muscle strength, intrinsic capacity | Improved strength and independence | Lack of unified frailty criteria |
| Dun et al. (2022) [11] | Randomized controlled trial | 48 pre-frail Chinese adults | X-CircuiT circuit training | 3 months, 46 min/session, 3×/week | Frailty prevalence, functional status | Pre-frailty reduced from 95% to 14%; NNT=1 | Small sample, single-center |
| Yao et al., 2024 [4] | Systematic review | RCTs with frailty subgroups | Various non-pharmacological interventions including exercise | Variable | Effect modification by frailty status | Frailty modifies intervention effectiveness | Few trials with stratified analysis |
| Ye et al., 2024 [12] | Randomized controlled trial | Older adults with cognitive frailty (n=120) | Baduanjin (mind–body exercise) | 24 weeks; 3×/week | Cognitive function, oxidative stress, inflammation | Improved cognition, reduced IL-6, CRP | Single-center, small sample |
| Fang et al. (2024) [13] | Randomized controlled trial | 144 pre-frail older adults | Multicomponent + motivational theory | 12 weeks, 3×/week | Frailty status, adherence | 68% frailty reversal vs. 25% controls; ↑ adherence | Requires trained facilitators |
| Fielding et al. (2024) [14] | Longitudinal clinical trial | Older adults, intervention groups | Structured exercise programs | Variable | Biomarkers, mobility | Reduced senescence biomarkers, improved mobility | Biomarker approaches not standardized |
| Huang et al. (2023) [15] | Systematic review & meta-analysis | Multidomain interventions (exercise + nutrition + cognition) | Combined multidomain programs | Variable | Frailty status | Significant improvements in frailty and independence | Protocol heterogeneity |
| Han et al. (2023) [16] | Pilot RCT | Pre-frail/frail hospitalized adults | Hospital-to-home exercise + nutrition | 12 weeks | Frailty scores, grip strength, cognitio | ↓ Frailty scores, ↑ grip strength & cognition | Pilot study, small scale |
SPPB — Short Physical Performance Battery; TUG — Timed-Up-and-Go test; QoL — Quality of Life; PA — Physical Activity; RCT — Randomized Controlled Trial.
Higher levels of physical activity are associated with an approximately 40–55% lower risk reduction in frailty odds, demonstrating a robust protective effect across diverse populations and assessment methods [3,9]. This protective relationship is consistent regardless of specific frailty assessment tools or activity indicators used, highlighting the fundamental importance of PA in maintaining physiological resilience in older adults.
A comprehensive analysis of exercise intervention characteristics provides guidance for optimal program design. Caicedo-Pareja et al. 2024 [8] reviewed 15 studies involving frail and pre-frail older adults, identifying key program features associated with successful outcomes. Multicomponent programs incorporating strength, balance, and aerobic elements for 8-24 weeks showed the most consistent benefits, with frequency of 2-3 sessions per week proving optimal for both safety and efficacy. The review emphasized the importance of progressive overload principles and individualized intensity prescription, particularly for frail populations where exercise tolerance varies considerably.
Multicomponent Exercise Programs (MEPs) are consistently highlighted as highly effective interventions for both pre-frail and frail older adults [5]. These programs significantly improve frailty status, enhance physical function (including measures like Short Physical Performance Battery (SPPB), grip strength, gait speed, and Timed-Up-and-Go (TUG) test results), boost cognitive performance, and contribute to improved emotional well-being. A systematic review and meta-analysis found that MEPs led to significant improvements in muscle strength, balance, endurance, and quality of life in frail elderly populations [6].
The most comprehensive recent meta-analysis provides definitive quantitative evidence for the effectiveness of multicomponent exercise in frailty prevention. Studies made by Sirikul et al. 2024 [9] conducted a systematic review and meta-analysis of 18 randomized controlled trials involving 3,457 community-dwelling older adults, examining the effects of multicomponent exercise and nutritional supplement interventions on frailty outcomes. The pooled analysis demonstrated that multicomponent exercise programs reduced frailty risk by 55% (95% CI: 45-67%), representing a large and clinically significant protective effect.
Community-based exercise interventions have demonstrated particular effectiveness in pre-frail older adults, significantly improving physical function, cognition, quality of life, and frailty status [5]. The efficacy of multicomponent exercises in improving physical frailty appears to be robust, regardless of the specific duration and types of activities, suggesting a degree of flexibility in program design [7]. However, highly structured and evidence-based programs that combine elements of strength, endurance, balance, and gait training in a tailored manner show consistent improvements in both physical and cognitive function.
Evidence for exercise interventions extends beyond community settings to institutionalized populations. A recent systematic review by Martínez-Montas et al. 2025 [10] analyzed 12 randomized trials involving 1,223 institutionalized older adults, finding that multicomponent exercise programs consistently improved frailty indicators and achieved frailty reversal in approximately 36% of participants. The review highlighted enhanced efficacy when exercise was combined with cognitive or nutritional components, supporting the value of integrated approaches in residential care settings where frailty prevalence is particularly high.
Recent evidence demonstrates that intensive, structured exercise programs can achieve dramatic reversal of pre-frailty in community settings. Dun et al. 2022 [11] conducted a randomized controlled trial of the Xiangya Hospital circuit training (X-CircuiT) program involving 48 pre-frail Chinese older adults. The intervention consisted of 46-minute sessions, three times per week for 3 months, incorporating resistance, balance, and aerobic components. Remarkably, pre-frailty prevalence decreased from 95% to 14% in the intervention group compared to controls, yielding an absolute risk reduction of 82% and a number needed to treat of 1. This study provides compelling evidence that high-frequency, supervised multicomponent programs can produce rapid and substantial improvements in frailty status.
Resistance Training (RT) can induce substantial increases in maximal strength (6.6% to 37%), muscle mass (3.4% to 7.5%), and muscle power (8.2%). RT improves functional capacity (4.7% to 58.1%) and significantly reduces the risk of falls. Given that sarcopenia (age-related loss of muscle mass and strength) is a key contributor to frailty, resistance training is considered one of the most effective measures to prevent and treat this condition [17].
Consistent engagement in aerobic activity has been shown to reduce the risk of frailty. Beyond its direct impact on frailty, aerobic exercise contributes to improved cardiorespiratory fitness and overall health, supporting the maintenance of physiological reserves essential for preventing frailty development [18].
Balance training is particularly important for fall prevention, a major adverse outcome of frailty. Recent systematic reviews confirm that physical training, especially multicomponent programs that include balance training or practices like Tai Chi, significantly improves balance [19].
Mind-body training modalities, such as Tai Chi and Baduanjin, have been found to significantly alleviate debilitating conditions and markedly improve quality of life, demonstrating superior efficacy over other physical activities in some analyses [12]. A randomized controlled trial demonstrated that 24 weeks of Baduanjin exercise significantly improved cognitive frailty while modulating oxidative stress and inflammatory processes, showing reduced pro-oxidative markers and increased antioxidant levels, along with beneficial changes in inflammatory cytokines [12].
The integration of behavioral theory with exercise interventions shows promise for enhancing long-term adherence and outcomes. Fang et al. 2024 [13] conducted a 24-week randomized trial incorporating Wellness Motivation Theory into a multicomponent exercise program for 144 pre-frail older adults. The intervention achieved pre-frailty reversal in 68.2% of participants compared to 25.4% in controls (absolute risk reduction of 42.8%), while simultaneously improving self-efficacy and exercise adherence. This study demonstrates that theoretically-grounded motivational components can enhance the effectiveness of exercise interventions beyond purely physiological benefits
Emerging research has identified cellular senescence as a key biological mechanism linking exercise to frailty prevention. Fielding et al. 2024 [14] demonstrated that biomarkers of cellular senescence predict mobility disability onset in older adults and are significantly reduced by physical activity interventions. Their longitudinal analysis revealed that exercise-induced reductions in senescence-associated secretory phenotype (SASP) markers correlated with improved physical function and delayed frailty progression. These findings provide mechanistic evidence for exercise as an anti-aging intervention at the cellular level.
Metabolomic profiling has revealed specific biochemical signatures associated with frailty that may serve as intervention targets. Pan et al. 2022 [20] identified distinct metabolomic patterns in frail older Chinese adults, including alterations in amino acid metabolism, lipid profiles, and energy metabolism pathways. These metabolomic signatures correlated with physical performance measures and frailty severity, suggesting that exercise interventions may exert their effects through restoration of metabolic homeostasis. Such biomarker approaches offer potential for personalized intervention strategies and objective monitoring of treatment responses.
Exercise-induced changes in musculoskeletal biomarkers provide insight into the mechanisms underlying frailty prevention. Abreu et al. 2022 [21] systematically reviewed biomarker responses to exercise in older adults, identifying consistent patterns in muscle protein synthesis markers, inflammatory cytokines, and bone metabolism indicators. Exercise interventions consistently elevated anabolic markers such as IGF-1 and reduced catabolic factors including myostatin, while simultaneously decreasing inflammatory markers associated with sarcopenia. These biomarker changes preceded and predicted functional improvements, supporting their utility as intermediate endpoints in frailty research.
The relationship between sarcopenia and cognitive decline highlights the importance of the muscle-brain axis in frailty development. Arosio et al. 2023 [22] reviewed evidence demonstrating bidirectional communication between skeletal muscle and brain through myokines, neurotrophic factors, and shared metabolic pathways. Exercise interventions that target both physical and cognitive domains show superior outcomes compared to single-domain approaches, supporting the concept of frailty as a systemic condition requiring comprehensive intervention. This muscle-brain crosstalk provides a biological rationale for multicomponent programs that include both physical and cognitive elements.
Chronic low-grade inflammation, often referred to as "inflammaging," is a recognized key contributor to the development and progression of frailty. Regular exercise programs have been consistently shown to significantly reduce levels of pro-inflammatory biomarkers, such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) [12]. This long-term anti-inflammatory effect is crucial for systemic health.
Interestingly, high-intensity exercise acutely triggers inflammation and reactive oxygen species (ROS) production. However, this acute, transient inflammatory response is distinct from chronic inflammation and is, in fact, essential for anti-aging benefits. It acts as an activator of adaptive responses in skeletal muscle, facilitating the clearance of senescent cells and promoting tissue repair and regeneration [23]. This "hormetic" effect, where a mild, controlled stressor induces a beneficial adaptive response, is a critical concept for understanding how exercise fundamentally improves cellular health.
Age-related hormonal dysregulation, such as increased cortisol levels, contributes to the pathophysiology of frailty. Exercise has been shown to positively influence hormonal balance. It can improve insulin resistance and stimulate the secretion of beneficial hormones associated with healthy aging, including growth hormone (GH), insulin-like growth factor 1 (IGF-1), and dehydroepiandrosterone sulfate (DHEA-S) [24]. These hormonal adaptations contribute to improved metabolic health and anabolic processes, further bolstering the body's resilience against frailty.
Exercise training has been shown to reduce oxidative stress markers and improve mitochondrial function in older adults [25]. The enhancement of mitochondrial biogenesis and function through regular physical activity helps maintain cellular energy production and reduces the accumulation of cellular damage associated with aging and frailty development [26].
The inherent heterogeneity in study populations and frailty definitions across research highlights the potential benefits of personalized approaches [7]. These tailored strategies, which may incorporate both exercise and nutritional interventions, hold promise for enhanced effectiveness in reducing frailty. Frailty status itself can serve as an effect modifier in interventions, with non-pharmacological interventions, including exercise programs, demonstrating greater benefits in patients with higher frailty levels [4].
Despite the compelling evidence, certain limitations in the current research warrant consideration. The persistent heterogeneity in frailty definitions and physical activity assessment methods across different studies continues to pose challenges for direct comparisons and the generalizability of findings [5,6]. Future research would benefit immensely from the establishment of more standardized approaches for frailty definition and physical activity measurement, which would enhance comparability and precision across studies [3].
Further investigation into the long-term effects of specific physical activity interventions is also needed, alongside studies exploring optimal dose-response relationships for diverse older adult populations, particularly those with varying comorbidities or from different cultural contexts [4,5]. Research should also continue to explore the synergistic effects of integrating physical activity with other interventions, such as nutrition and social engagement [5]. Expanding studies to include low- and middle-income countries is crucial to broaden the applicability of findings and address global health disparities [3].
The integration of digital health technologies represents an emerging frontier in frailty prevention. Li et al. 2024 [27] describe the PF-Life protocol, a comprehensive mHealth platform-based lifestyle-integrated multicomponent exercise program designed to reverse pre-frailty in community-dwelling older adults. This innovative 12-month randomized controlled trial will evaluate not only functional outcomes but also inflammatory biomarkers including IL-6, TNF-α, and CRP, providing mechanistic insights into exercise-induced frailty reversal. The digital delivery model addresses scalability challenges while maintaining intervention fidelity through remote monitoring and personalized feedback systems.
Recognition of exercise limitations in some older adults has prompted investigation of pharmacological adjuncts to physical activity interventions. The MET-PREVENT trial protocol by Rennie et al. 2022 [28] describes a double-blind randomized study testing metformin to improve physical performance in older adults with sarcopenia and physical prefrailty/frailty. This approach targets metabolic pathways implicated in aging and frailty, potentially offering alternatives when exercise alone is insufficient or contraindicated. Such pharmacological strategies may complement exercise interventions or provide bridging therapy for individuals unable to participate in intensive physical activity programs.
Future therapeutic approaches may combine exercise with pro-myogenic compounds to enhance intervention efficacy. Storer et al. 2023 [29] outlined the potential for exercise to serve as an adjunct to emerging pharmacological treatments targeting muscle protein synthesis and mitochondrial function. This combined approach recognizes that while exercise remains the cornerstone intervention, additional therapeutic modalities may be necessary to achieve optimal outcomes in severely frail individuals or those with limited exercise capacity. Such combination therapies represent the next frontier in frailty prevention and treatment.
The effectiveness of physical activity within multidomain interventions has been further validated in recent controlled trials. Huang et al. 2023 [15] implemented a 12-week program combining health education, cognitive training, and exercise in Taiwanese older adults, demonstrating significant improvements in frailty status. Importantly, their analysis revealed strong correlations between increased physical activity levels and improvements in mobility, cognitive performance, and mood, reinforcing the central role of exercise within comprehensive frailty prevention strategies. This multidomain approach achieved frailty improvements while simultaneously addressing multiple age-related domains.
Evidence from the past five years consistently demonstrates that physical activity is a powerful and modifiable factor capable of preventing, attenuating, and even reversing frailty in older adults [3–6]. Meta-analytical data indicate that individuals with higher levels of regular physical activity have approximately 40–55% lower odds of developing frailty compared to sedentary peers [3,9].
The mechanisms underlying this protective effect are multifaceted, involving structural and functional adaptations in the musculoskeletal, cardiovascular, metabolic, and neurocognitive systems. Regular exercise increases muscle mass and strength by 5–10%, improves gait speed by up to 20%, and enhances balance and coordination, thereby reducing the risk of falls and dependence [5–7]. It also improves mitochondrial efficiency, reduces oxidative stress and inflammatory biomarkers such as IL-6 and CRP, and supports anabolic hormonal balance, including growth hormone and IGF-1 activity [12,23,24].
Translation of research evidence into clinical practice requires clear guidelines for healthcare providers. Studies [30] developed comprehensive clinical practice guidelines for nutrition and physical activity in older adults living with frailty, emphasizing individualized assessment and progressive intervention strategies. The guidelines recommend multicomponent exercise programs as first-line treatment, with specific modifications for different frailty stages and comorbidity profiles. These evidence-based recommendations provide practical frameworks for implementing exercise interventions in diverse clinical settings
Among different interventions, multicomponent exercise programs combining aerobic, resistance, and balance training demonstrate the most consistent benefits, significantly improving frailty status, physical performance (SPPB, TUG), and cognitive outcomes [5,6]. Importantly, these effects are observed both in pre-frail and frail individuals, indicating the reversibility of frailty when targeted exercise is applied early and consistently.
The INDEPENDENCE randomized controlled pilot trial[16] evaluated an individualized hospital-to-home exercise-nutrition self-management program achieving excellent adherence rates exceeding 90%. Participants showed greater reductions in Edmonton Frail Scale scores at 3 months (-3.0; 95% CI: -4.8 to -3.0) and 6 months (-2.5; 95% CI: -3.8 to -1.0) compared to controls, plus improvements in handgrip strength (+3.7 kg) and cognitive function. This demonstrates that self-managed combined interventions can achieve meaningful frailty reversal in high-risk hospitalized populations
From a clinical perspective, physical activity should not be regarded merely as a general lifestyle recommendation but as a therapeutic intervention with proven efficacy. Healthcare professionals should assess baseline activity levels and prescribe individualized exercise programs aligned with frailty severity and functional capacity.
On a public health level, the implementation of structured physical activity programs can substantially reduce healthcare costs and the societal burden of aging. A 10–15% improvement in physical function across populations could translate into measurable reductions in hospital admissions and long-term care needs. This highlights the necessity for integrating exercise-based prevention into national aging and rehabilitation strategies, supported by dedicated funding and professional education in exercise prescription for older adults.
Regular physical activity is a key determinant of healthy aging and one of the most effective non-pharmacological interventions for preventing and reversing frailty in older adults. The accumulated evidence from recent systematic and narrative reviews demonstrates that higher activity levels reduce the risk of frailty by approximately 40-55%, while structured and individualized exercise programs can improve muscle strength, gait speed, balance, and cognitive performance.
Among available modalities, multicomponent exercise programs that combine resistance, aerobic, and balance training consistently provide the strongest functional and physiological benefits, supporting the concept of frailty as a potentially reversible condition. Their effects are mediated through the mitigation of sarcopenia, reduction of chronic inflammation and oxidative stress, enhancement of mitochondrial efficiency, and optimization of hormonal regulation.
Physical activity should therefore be recognized as a therapeutic strategy, not only a preventive measure. Routine assessment of physical activity and individualized exercise prescriptions should become an integral component of geriatric care and rehabilitation.
Future research should focus on establishing standardized definitions of frailty and physical activity assessment, determining optimal dose–response relationships for diverse populations, and integrating physical activity with nutritional and psychosocial interventions. Expanding evidence from low- and middle-income countries remains essential for achieving global applicability of current recommendations.
By prioritizing physical activity as a cornerstone of geriatric medicine, healthcare systems can significantly reduce the burden of frailty, preserve autonomy in aging populations, and improve both individual and societal health outcomes.
Jan Drugacz – conceptualization, methodology, writing – original draft, editing.
Olaf Helbig – literature search, data extraction, writing – review & editing.
Bartosz Bobrowski – data interpretation, clinical insights, review of manuscript.
Honorata Derlatka – methodology, supervision, manuscript editing.
Klaudia Kontek – literature search, drafting sections, reference verification.
Anna Kukhtiak – manuscript preparation, formatting, data validation.
Julia Marcinkowska – data extraction, figures and tables preparation.
Martyna Radelczuk – writing – review & editing, critical revision.
Łukasz Skowron – supervision, clinical expertise, final approval of the manuscript.
All authors have read and approved the final version of the manuscript.
Artificial intelligence tools were not used in the writing or editing of this manuscript.