- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 5. DOI 10.35630/2025/15/Iss.5.504
Background: Oxidative stress, resulting from an imbalance between reactive oxygen species (ROS) and antioxidant defenses, is a central mechanism implicated in the development of cardiovascular, metabolic, neurodegenerative, pulmonary, and oncological diseases. Despite decades of research, the clinical translation of antioxidant strategies remains controversial, with large trials producing inconsistent results and leaving key questions unresolved.
Aims: This narrative review aimed to synthesize current evidence on the role of oxidative stress in chronic disease pathogenesis, to elucidate underlying molecular mechanisms, to critically assess the effectiveness of antioxidant and lifestyle interventions, and to identify unresolved controversies, knowledge gaps, and future research priorities.
Methods: A narrative literature review with systematic search elements was performed in PubMed, Scopus, and Web of Science for studies published between January 2010 and July 2025. From 300 records initially identified, 71 studies met the inclusion criteria and were included in the analysis. Data were extracted on disease associations, oxidative stress pathways, therapeutic and lifestyle interventions, and clinical outcomes. The methodological quality of included studies was assessed using adapted Newcastle–Ottawa and AMSTAR-2 criteria.
Results: Evidence supports oxidative stress as a unifying but heterogeneous mechanism across chronic diseases. Strong associations are documented in cardiovascular and metabolic disorders, whereas findings in neurodegeneration and cancer remain inconsistent. Antioxidant supplementation demonstrates biological plausibility in preclinical models but has yielded conflicting clinical outcomes due to poor bioavailability, population heterogeneity, and methodological limitations. Lifestyle interventions, including dietary modification, physical activity, and smoking cessation, consistently reduce oxidative stress markers and remain the most evidence-based strategies. Novel therapeutic approaches, such as mitochondria-targeted antioxidants, gene therapy, and nanotechnology-based delivery systems, are promising but lack validation in large-scale clinical studies.
Conclusions: Oxidative stress is a well-established pathogenic factor across chronic diseases, but its therapeutic modulation remains unproven in clinical practice. Current antioxidant therapies cannot be recommended for routine use, whereas lifestyle modifications provide reproducible benefits. Future research should focus on standardized biomarkers, rigorously designed long-term clinical trials, and precision strategies to establish the clinical relevance of oxidative stress management.
Keywords: Oxidative stress, reactive oxygen species, chronic diseases, antioxidants, cardiovascular disease, diabetes, neurodegenerative disorders, cancer, COPD
Oxidative stress occurs when the production of reactive oxygen species (ROS), such as superoxide (O₂⁻), hydrogen peroxide (H₂O₂), and hydroxyl radicals (OH⁻), overwhelms the body’s antioxidant defense mechanisms, including enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [9]. ROS are primarily generated in mitochondria during oxidative phosphorylation but can also arise from external factors such as cigarette smoke, air pollution, and ultraviolet radiation [10]. At physiological levels, ROS play critical roles in cell signaling, immune responses, and homeostasis; however, excessive ROS production leads to oxidative damage to lipids, proteins, and DNA, contributing to the pathogenesis of numerous chronic diseases [11]. Conditions such as cardiovascular diseases (CVD), type 2 diabetes mellitus (T2DM), neurodegenerative disorders (e.g., Alzheimer’s and Parkinson’s diseases), chronic obstructive pulmonary disease (COPD), and cancer have been consistently linked to oxidative stress [12,13].
Although oxidative stress is widely recognized as a central mechanism in chronic disease pathogenesis, the literature remains divided on the clinical utility of antioxidant therapies. Several large clinical trials have reported limited or inconsistent benefits of antioxidant supplementation, raising debate about their therapeutic relevance and translational value [14,15,16,26,55].
Furthermore, significant gaps in knowledge persist regarding disease-specific ROS signaling pathways, the bioavailability and pharmacokinetics of antioxidant compounds, and the long-term outcomes of emerging interventions [59,60,61,62]. These controversies and uncertainties highlight the need for a narrative review that not only synthesizes recent evidence but also critically examines conflicting results and identifies directions for future research [63,64].
Despite decades of investigation, the clinical translation of antioxidant strategies remains controversial, and recent studies have yielded inconsistent results [6,7,22,23,24,26]. Previous reviews have often focused on single disease groups or isolated compounds, whereas a comparative synthesis across major chronic conditions is still lacking [3,4,25]. Advances in redox biology, including insights into mitochondrial ROS signaling and redox-sensitive transcription factors, provide new perspectives that warrant integration [59,67,70]. Given the continuing global burden of chronic diseases, this review seeks to address current knowledge gaps by synthesizing recent evidence, highlighting unresolved controversies, and outlining future research priorities for oxidative stress-related interventions [65,66,68,69].
This work was conducted as a narrative literature review with systematic search elements. The aim was to provide a broad synthesis of current knowledge while applying a structured search strategy to ensure coverage of the most relevant studies.
A literature search was performed in PubMed, Scopus, and Web of Science for publications between January 2010 and July 2025. The search terms included combinations of the following keywords: “oxidative stress,” “reactive oxygen species,” “chronic diseases,” “cardiovascular disease,” “diabetes,” “neurodegenerative disorders,” “chronic obstructive pulmonary disease,” “cancer,” and “antioxidants.” Boolean operators (AND/OR) were applied to refine the search. Filters were set to include only peer-reviewed original articles and reviews published in English.
In total, 300 records were identified. After removing duplicates, titles and abstracts were screened for relevance. Full texts were then reviewed according to predefined criteria:
Following this process, 71 studies were retained for analysis. While this number does not reflect the entirety of published work on oxidative stress, it was considered sufficient to cover the major disease groups and therapeutic directions addressed in this review.
Data were extracted regarding study design, disease focus, oxidative stress mechanisms, therapeutic or lifestyle interventions, and reported clinical outcomes. The methodological quality of included studies was assessed using adapted criteria from the Newcastle–Ottawa Scale for clinical and observational research, and AMSTAR-2 for review articles. Parameters considered included study design rigor, sample size adequacy, and statistical robustness.
Although elements of the PRISMA framework were used to guide study selection, this review does not fully meet the requirements of a systematic review. Therefore, results are presented as a narrative synthesis supported by a structured search strategy. A simplified PRISMA-style flow diagram of the selection process is provided in Supplementary Figure 1.
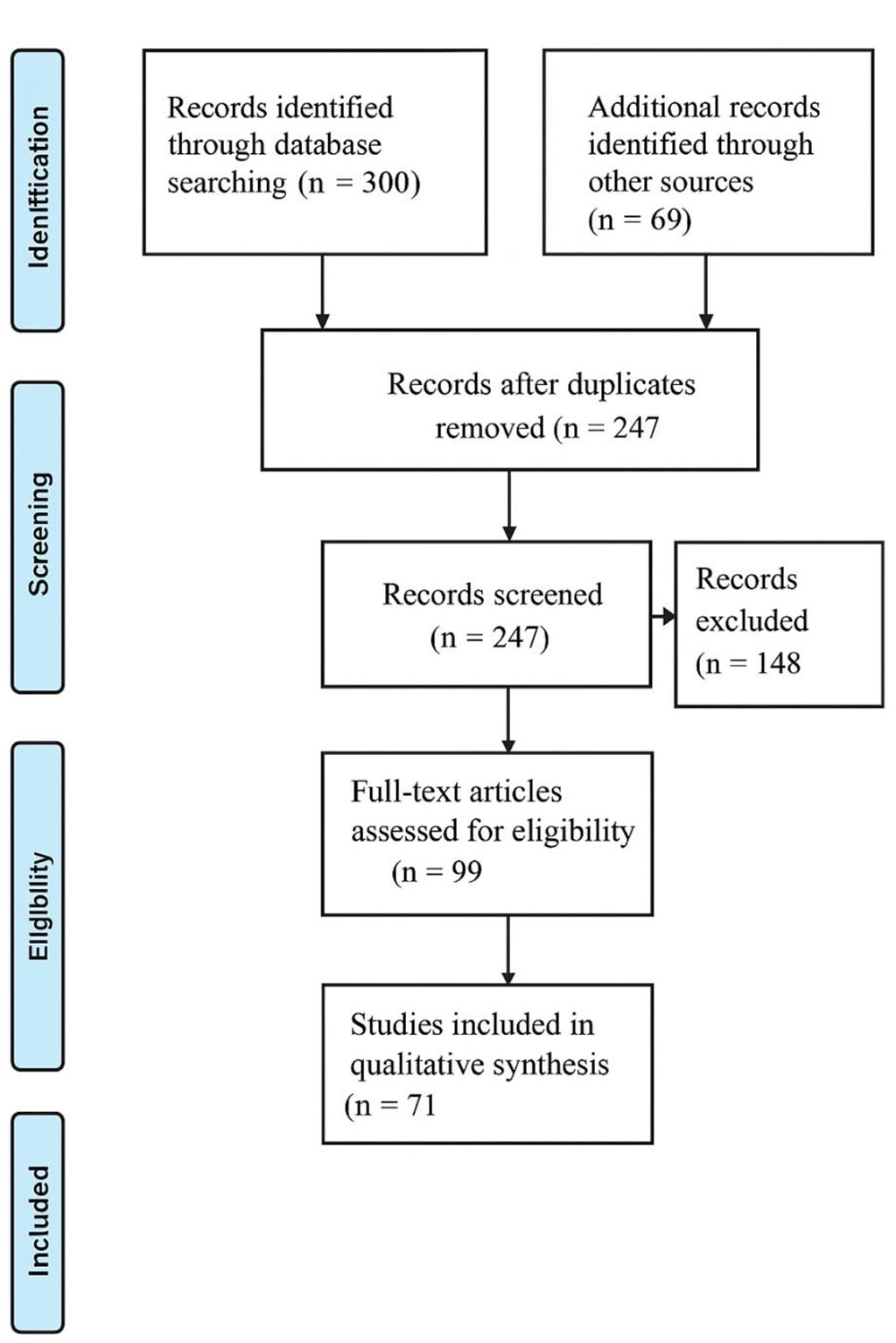
Figure 1. PRISMA-style flow diagram of the literature selection process
Flow diagram illustrates the process of study selection for the review. A total of 300 records were identified through database searching. After removal of duplicates (n = 247), titles and abstracts were screened, resulting in 148 exclusions. Subsequently, 99 full-text articles were assessed for eligibility, with 28 excluded for not meeting inclusion criteria. Finally, 71 studies were included in the qualitative synthesis of this review.
The selected studies included experimental research, clinical trials, and systematic reviews, providing a comprehensive evidence base. The findings consistently demonstrated that oxidative stress is a central pathological mechanism in chronic diseases, with ROS contributing to cellular damage, inflammation, and dysregulated signaling pathways. Key mechanisms included lipid peroxidation, protein oxidation, DNA damage, and activation of pro-inflammatory pathways such as nuclear factor-kappa B (NF-κB). Antioxidant therapies, including dietary antioxidants and pharmacological agents, showed variable efficacy, with some studies reporting significant reductions in oxidative damage and others highlighting limitations due to poor bioavailability, non-specific targeting, or advanced disease stages [14,15]. Emerging therapies, such as mitochondria-targeted antioxidants and gene therapy, were identified as promising areas for future research. To provide a concise overview of the fundamental principles and pathogenic implications of oxidative imbalance, Table 1 summarizes the key aspects of oxidative stress, including its main sources, exogenous triggers, antioxidant defense systems, and clinical relevance to major disease groups [1–15].
Table 1. Key aspects of oxidative stress in chronic diseases
| Aspect | Description | References |
| Definition | Imbalance between reactive oxygen species (ROS) production and antioxidant defense systems. | [1-3] |
| Main ROS | Superoxide (O₂⁻), hydrogen peroxide (H₂O₂), hydroxyl radical (OH⁻). | [2-4] |
| Primary sources | phosphorylation, NADPH oxidase, xanthine oxidase. | [3,5] |
| Exogenous triggers | Cigarette smoke, air pollution, UV radiation, certain drugs and toxins. | [6,7] |
| Antioxidant defense systems | Enzymatic: SOD, CAT, GPx; Non-enzymatic: vitamins C/E, glutathione, flavonoids. | [8-10] |
| Pathological consequences | Lipid peroxidation, protein oxidation, DNA damage, mitochondrial dysfunction. | [11,12] |
| Clinical relevance | T2DM, neurodegeneration, COPD, cancer. | [13-15] |
| Therapeutic implications | Antioxidant supplementation, lifestyle modification, emerging targeted therapies. | [16-20] |
Cardiovascular diseases (CVDs), a group encompassing clinical entities such as atherosclerosis, arterial hypertension, and heart failure, continue to represent one of the foremost global health challenges, being responsible for a significant proportion of morbidity and mortality on a worldwide scale [16] A growing body of scientific evidence underscores the pivotal role played by oxidative stress in the initiation and progression of these pathological conditions, particularly through its deleterious impact on vascular homeostasis.
One of the central mechanisms by which oxidative stress contributes to cardiovascular pathology is via the induction of endothelial dysfunction, which constitutes a fundamental and early event in the pathogenesis of atherosclerosis. In this context, the excessive production of reactive oxygen species (ROS) leads to the oxidative modification of lipids, most notably resulting in the formation of oxidized low-density lipoprotein (ox-LDL). This oxidized lipoprotein form plays a critical role in the atherogenic process by being taken up by macrophages, thereby initiating foam cell formation and fostering the development of atherosclerotic plaques [17].
Moreover, ROS serve as potent activators of multiple intracellular pro-inflammatory signaling pathways. Of particular relevance is the activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which regulates the expression of various genes involved in inflammation. This includes upregulation of adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), as well as pro-inflammatory cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), all of which collectively amplify vascular inflammation and promote plaque instability [18].
In the setting of hypertension, oxidative stress plays a similarly detrimental role by interfering with the bioavailability of nitric oxide (NO), a vasodilatory molecule essential for the maintenance of vascular tone and endothelial function. ROS rapidly react with NO, leading to the formation of peroxynitrite and diminishing NO-mediated vasodilation, which in turn increases peripheral vascular resistance and contributes to the maintenance of elevated blood pressure levels [19]. A key enzymatic source of ROS in vascular cells is NADPH oxidase, whose activity has been found to be significantly upregulated in patients with hypertension, further aggravating oxidative imbalance and facilitating disease progression [20].
In the case of heart failure, recent investigations have highlighted the involvement of mitochondrial-derived ROS in the deterioration of cardiac function. These reactive species compromise the integrity of cardiomyocytes by inducing apoptotic pathways and promoting fibrotic remodeling of cardiac tissue, both of which contribute to the progressive decline in myocardial performance observed in heart failure syndromes [21].
In response to the recognition of oxidative stress as a major contributor to cardiovascular pathology, several antioxidant strategies have been explored in both experimental and clinical settings. Among these, supplementation with compounds such as coenzyme Q10, vitamin E, and naturally occurring polyphenols derived from sources like green tea and red wine has shown potential in ameliorating oxidative damage. These interventions have been associated with improvements in endothelial function, attenuation of atherosclerotic processes, and reductions in systemic blood pressure [22,23]. For instance, a well-designed randomized controlled trial demonstrated that coenzyme Q10 administration resulted in a statistically significant decrease in systolic blood pressure in individuals diagnosed with hypertension, supporting its therapeutic utility in clinical practice [24].
Beyond pharmacological and nutraceutical approaches, lifestyle modifications have also been recognized as effective means of modulating oxidative stress in patients with cardiovascular disease. Regular aerobic physical activity, in particular, has been shown to enhance the activity of endogenous antioxidant enzymes, thereby strengthening the body's defense mechanisms against ROS-induced damage and contributing to overall cardiovascular health improvement [25].
In conclusion, oxidative stress plays a multifaceted and central role in the etiology and progression of cardiovascular diseases. Its influence extends across various pathological processes, including endothelial dysfunction, vascular inflammation, impaired vasodilation, and myocardial injury. Antioxidant therapies and lifestyle interventions offer promising avenues for mitigating the impact of oxidative stress, although further research is warranted to establish standardized protocols and determine their long-term efficacy across diverse patient populations.
Type 2 diabetes mellitus (T2DM) is a complex and multifactorial metabolic disorder primarily characterized by the presence of insulin resistance, persistent hyperglycemia, and a state of chronic, low-grade systemic inflammation. These pathophysiological features are closely intertwined with increased oxidative stress, which not only accompanies the disease process but also actively contributes to its initiation and progression [26].
A sustained elevation of blood glucose levels, typical of poorly controlled T2DM, leads to enhanced production of reactive oxygen species (ROS) through a variety of interconnected biochemical pathways. Among the most prominent mechanisms are the polyol pathway, which consumes NADPH and diminishes the cellular antioxidant capacity; the hexosamine biosynthetic pathway, which alters protein function via aberrant glycosylation; activation of protein kinase C (PKC), which disrupts normal cellular signaling; and the non-enzymatic glycation of proteins and lipids, culminating in the formation of advanced glycation end-products (AGEs) [27]. The cumulative effect of these pathways results in increased oxidative burden at the cellular and systemic levels.
These molecular processes ultimately impair the viability and functionality of pancreatic β-cells, which are particularly susceptible to oxidative damage due to their inherently low expression of antioxidant enzymes. The ensuing β-cell dysfunction manifests as a reduction in insulin biosynthesis and secretion, thereby exacerbating hyperglycemia and perpetuating the cycle of oxidative stress. Furthermore, the oxidative environment contributes significantly to the development and progression of diabetes-related complications, including but not limited to diabetic nephropathy, retinopathy, peripheral neuropathy, and various forms of cardiovascular pathology [28].
Oxidative stress not only results from hyperglycemia but also intensifies its deleterious effects through the promotion of AGE formation. These AGEs, in turn, interact with specific cell surface receptors known as RAGE (receptors for advanced glycation end-products), initiating a cascade of intracellular signaling events that culminate in the activation of inflammatory transcription factors, such as NF-κB. This process leads to the upregulation of pro-inflammatory cytokines and adhesion molecules, thereby exacerbating endothelial dysfunction and accelerating vascular injury in diabetic individuals [29].
A growing body of clinical research has examined the therapeutic potential of antioxidant compounds in the management of T2DM. Notably, substances such as alpha-lipoic acid, N-acetylcysteine, and vitamin C have demonstrated the ability to improve insulin sensitivity and mitigate oxidative damage in patients diagnosed with this condition [30,31]. For example, evidence from a meta-analysis indicates that supplementation with alpha-lipoic acid is associated with statistically significant improvements in glycemic control, as well as a reduction in oxidative stress biomarkers among diabetic populations [32].
In addition to pharmacological supplementation, dietary modifications that emphasize the intake of natural antioxidants have shown promise in improving clinical outcomes in T2DM. Specifically, adherence to the Mediterranean diet characterized by high consumption of fruits, vegetables, whole grains, legumes, olive oil, and fatty fish has been associated with enhanced glycemic control and a reduction in cardiovascular risk factors in individuals with type 2 diabetes [33].
However, despite these encouraging findings, results from some clinical trials investigating antioxidant interventions remain inconsistent. In certain studies, the observed benefits have been limited or absent, which may be attributable to heterogeneity among study populations, differences in disease duration, variability in the formulations and dosages of antioxidants used, or issues related to bioavailability and pharmacokinetics of the compounds in question [34].
Neurodegenerative disorders, encompassing conditions such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS), are complex, progressive pathologies characterized by the gradual loss of structure and function of neurons. A growing body of evidence implicates oxidative stress as a central mechanism in the pathogenesis and advancement of these diseases, wherein excessive production of reactive oxygen species (ROS) leads to cumulative neuronal damage and eventual cell death [35].
In Alzheimer’s disease, one of the most prevalent forms of dementia, oxidative stress is intricately linked to amyloid-beta (Aβ) pathology. The aggregation and deposition of Aβ peptides into extracellular plaques is known to stimulate ROS generation, which in turn causes profound mitochondrial dysfunction. The resulting bioenergetic deficits and oxidative damage compromise synaptic integrity and lead to apoptosis of neurons, particularly within the hippocampus and cerebral cortex regions critical for memory and cognition [36]. Furthermore, oxidative stress facilitates the hyperphosphorylation of tau protein, a microtubule-associated protein, thereby promoting the formation of neurofibrillary tangles another histopathological hallmark of AD that disrupts neuronal cytoskeletal stability and accelerates neurodegeneration [37].
In Parkinson’s disease, the role of oxidative stress is similarly profound. The degeneration of dopaminergic neurons in the substantia nigra pars compacta, which underlies the cardinal motor symptoms of the disease, is strongly associated with increased oxidative burden. Dopamine itself is a redox-active neurotransmitter, and its oxidative metabolism can yield ROS and quinones that damage cellular macromolecules. In addition, oxidative stress contributes to the misfolding and aggregation of alpha-synuclein, leading to the formation of Lewy bodies cytoplasmic inclusions characteristic of PD pathology [38].
Amyotrophic lateral sclerosis, a fatal neurodegenerative disease affecting upper and lower motor neurons, also demonstrates a compelling connection to oxidative stress. In ALS, mutations in the gene encoding superoxide dismutase 1 (SOD1), an enzyme responsible for detoxifying superoxide radicals, result in the accumulation of misfolded protein species and impaired mitochondrial function. These pathological changes disrupt neuronal homeostasis, increase oxidative load, and culminate in the progressive degeneration of motor neurons [39].
In light of these mechanistic insights, a considerable number of preclinical investigations have examined the neuroprotective potential of antioxidant compounds. Substances such as resveratrol, curcumin, and melatonin have demonstrated efficacy in experimental models by mitigating oxidative damage through various mechanisms. These include direct ROS scavenging, suppression of pro-inflammatory mediators, and preservation of mitochondrial integrity [40,41]. Notably, curcumin has shown the ability to attenuate Aβ-induced oxidative injury and cognitive deficits in transgenic mouse models of AD, highlighting its therapeutic promise [42].
Despite encouraging results from animal studies, the translation of antioxidant therapies into clinical benefit for patients with neurodegenerative diseases has yielded mixed and often inconclusive outcomes. Some clinical trials have observed modest symptomatic improvement or biomarker reduction, whereas others have failed to demonstrate statistically significant effects. These discrepancies may be attributable to several factors, including variability in disease stage among participants, differences in treatment duration and dosage, as well as limited bioavailability of antioxidants. One particularly formidable barrier is the blood-brain barrier (BBB), which restricts the penetration of many therapeutic agents into the central nervous system, thereby limiting their neuroprotective efficacy [43].
In response to these challenges, emerging strategies are being developed to enhance antioxidant delivery to neural tissues. Among the most promising are nanoparticle-based systems, which offer improved solubility, stability, and targeted delivery capabilities. Such advanced delivery platforms hold potential to overcome the pharmacokinetic limitations associated with conventional antioxidant therapies and may pave the way for more effective treatment modalities in neurodegenerative diseases [44].
Given the pivotal role of oxidative stress in the pathogenesis of neurodegenerative disorders, numerous studies have investigated its molecular mechanisms and therapeutic implications.
Table 2 summarizes the principal oxidative pathways, neuropathological consequences, and current therapeutic approaches described for major neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and Huntington’s disease [21–38]. This overview integrates experimental and clinical findings, highlighting the interplay between mitochondrial dysfunction, protein aggregation, excitotoxicity, and redox imbalance that collectively drive neuronal degeneration across these conditions.
Table 2. Oxidative stress mechanisms and therapeutic approaches in major neurodegenerative diseases
| Disease | Key oxidative stress mechanisms | Pathological consequences | Therapeutic approaches | Limitations/challenges | References |
| Alzheimer’s Disease (AD) | Mitochondrial dysfunction, Aβ-induced ROS, metal ion imbalance, impaired antioxidant enzymes | Neuronal loss, synaptic dysfunction, cognitive decline | Antioxidants (vitamin E, coenzyme Q10), lifestyle interventions, flavonoids | Poor
bioavailability, inconsistent clinical trial outcomes |
[21–25] |
| Parkinson’s Disease (PD) | Dopaminergic auto-oxidation, mitochondrial complex I dysfunction, α-synuclein aggregation | Nigrostriatal degeneration, motor dysfunction | Antioxidants (N-acetylcysteine, creatine), mitochondrial protectants | Limited translation from preclinical to clinical studies, small sample sizes | [26–30] |
| Amyotrophic Lateral Sclerosis (ALS) | Mutant SOD1-mediated ROS, glutamate excitotoxicity, mitochondrial damage | Motor neuron degeneration, progressive paralysis | Edaravone, experimental antioxidant therapies | Modest clinical benefit, short-term efficacy, lack of long-term validation | [31–34] |
| Huntington’s disease (HD) | Impaired mitochondrial respiration, mutant huntingtin-induced ROS | Striatal
neuronal loss, motor and cognitive decline |
Coenzyme
Q10, creatine, polyphenols |
No
proven clinical efficacy, heterogeneous patient response |
[35–38] |
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory condition characterized by irreversible airflow limitation, chronic inflammation of the airways, and a sustained imbalance between oxidants and antioxidants. One of the central pathogenic mechanisms implicated in the development and progression of COPD is oxidative stress, which arises primarily as a consequence of long-term exposure to exogenous noxious stimuli, most notably cigarette smoke and various environmental pollutants [45]. These harmful agents serve as abundant sources of reactive oxygen species (ROS), which overwhelm endogenous antioxidant defense systems and precipitate extensive molecular and cellular damage within pulmonary tissues.
Cigarette smoke, in particular, represents one of the most potent external contributors to oxidative burden in the lungs. It contains a wide array of free radicals and oxidant species capable of directly inducing injury to the bronchial epithelium. Such exposure leads to epithelial cell damage, increased mucous production, and structural alterations of the airways, collectively referred to as airway remodeling an essential feature of COPD pathology [46]. The sustained presence of ROS further aggravates mucociliary dysfunction and promotes the infiltration of inflammatory cells, thereby establishing a chronic inflammatory microenvironment within the respiratory tract.
The molecular pathways activated by oxidative stress in COPD are diverse and complex. Among the most critical is the nuclear factor kappa B (NF-κB) signaling pathway, which is sensitive to redox imbalance and becomes aberrantly activated in response to excessive ROS levels. Activation of NF-κB leads to the transcriptional upregulation of numerous pro-inflammatory genes, including those encoding cytokines such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α). These mediators play key roles in the recruitment and activation of neutrophils and macrophages, thereby perpetuating the cycle of inflammation and tissue injury [47].
A notable feature observed in patients suffering from COPD is the depletion of intrinsic antioxidant defense mechanisms. Specifically, the activity of enzymatic antioxidants such as superoxide dismutase (SOD) and glutathione peroxidase (GPx) is markedly diminished, thereby reducing the lung’s capacity to neutralize harmful oxidant species. This enzymatic insufficiency contributes to an environment favoring oxidative stress and exacerbates the structural and functional decline of the respiratory system [48].
More recent studies have brought attention to the role of mitochondria as endogenous sources of ROS in COPD. Mitochondrial dysfunction within airway epithelial cells has been shown to increase intracellular ROS production and promote apoptotic pathways. This mitochondrial-derived oxidative stress further compromises epithelial integrity and plays a critical role in the chronic progression of the disease [49].
In response to the central involvement of oxidative mechanisms in COPD, various antioxidant-based therapeutic strategies have been explored. Pharmacologic agents such as N-acetylcysteine and erdosteine have demonstrated efficacy in clinical trials, showing a reduction in the frequency and severity of COPD exacerbations, as well as improvements in lung function parameters. These compounds act by replenishing intracellular glutathione levels and directly scavenging free radicals, thereby mitigating oxidative injury [50].
In addition to pharmacologic interventions, dietary antioxidants have also been investigated for their potential to modulate oxidative stress in COPD patients. Nutrients such as vitamin C, vitamin E, and polyphenolic compounds, including flavonoids, have been associated with improved pulmonary function and reductions in oxidative biomarkers. Their beneficial effects are believed to stem from their capacity to neutralize ROS and attenuate inflammatory responses within the airway epithelium [51].
Beyond pharmacologic and nutritional approaches, pulmonary rehabilitation programs represent a multidisciplinary strategy incorporating structured exercise regimens and behavioral modifications, including smoking cessation. Participation in such programs has been shown to enhance the endogenous antioxidant capacity and reduce oxidative burden by decreasing systemic inflammation and promoting more efficient oxygen utilization during physical activity [52].
Oxidative stress plays a complex and multifaceted role in the pathogenesis and progression of cancer, functioning both as a driving force behind tumorigenesis and as a potential point of therapeutic intervention [53]. The excessive generation of reactive oxygen species (ROS) contributes significantly to the initiation of malignancy by promoting oxidative damage to cellular macromolecules, particularly DNA. This oxidative insult results in a spectrum of genetic alterations, including point mutations, chromosomal aberrations, and DNA strand breaks, which collectively contribute to genomic instability and ultimately foster malignant transformation of normal cells [54].
Cancer cells are often characterized by a persistently elevated oxidative state, a phenomenon attributed to their increased metabolic activity, mitochondrial dysfunction, and alterations in redox signaling pathways. The heightened levels of ROS within tumor cells are not merely byproducts of altered metabolism but serve as essential mediators of various oncogenic processes. These include the promotion of cellular proliferation, the stimulation of neovascularization through angiogenic signaling, and the facilitation of tissue invasion and metastasis. Despite their pro-tumorigenic functions, the elevated ROS milieu renders cancer cells inherently vulnerable to further increases in oxidative stress, presenting a unique therapeutic window [55].
This vulnerability has been strategically exploited through pro-oxidant therapies, which aim to induce cytotoxic levels of oxidative stress selectively in malignant cells. Agents such as high-dose vitamin C, anthracyclines, and ionizing radiation function by exacerbating oxidative stress beyond the adaptive threshold of cancer cells, leading to the activation of cell death pathways, including apoptosis and necrosis [56]. These treatments take advantage of the relatively lower antioxidant reserves of cancer cells compared to their normal counterparts, thereby achieving selective toxicity.
Conversely, antioxidants have been investigated for their capacity to prevent the initiation and progression of cancer by mitigating oxidative DNA damage and suppressing inflammation-driven carcinogenesis. However, the use of antioxidant supplementation in oncology remains controversial. While antioxidants may confer protective effects in the early stages of carcinogenesis, there is growing concern that, in established tumors, they may inadvertently shield malignant cells from oxidative damage induced by therapeutic interventions, thus impairing treatment efficacy and potentially facilitating tumor survival and progression [57]. This duality underscores the complexity and context-dependent nature of antioxidant use in oncology.
Recent advances have focused on the development of mitochondria-targeted antioxidants, such as MitoQ, designed to modulate ROS levels specifically within cancer cell mitochondria. These agents offer the potential to restore redox homeostasis in a manner that selectively impairs tumor cell viability while sparing normal cells. Preclinical investigations have yielded encouraging results, demonstrating reduced tumor growth and enhanced sensitivity to conventional therapies in models treated with mitochondria-directed antioxidants [58].
Furthermore, the tumor microenvironment (TME) has emerged as a critical modulator of oxidative stress dynamics in cancer. Characterized by regions of hypoxia, nutrient deprivation, and chronic inflammation, the TME sustains a state of oxidative imbalance that promotes cancer progression and contributes to therapeutic resistance. ROS produced within the TME influence numerous aspects of tumor biology, including angiogenesis, immune evasion, and metastasis. Consequently, targeting redox pathways within the TME represents a promising and rapidly evolving area of cancer research, with potential to enhance the efficacy of current therapeutic strategies and overcome resistance mechanisms [59,60].
Antioxidant therapies have garnered substantial attention in recent decades as a potential strategy for mitigating oxidative stress and its pathological consequences in a wide array of chronic diseases. Among the most extensively studied interventions are dietary antioxidants, particularly vitamins C and E, which have been the subject of numerous clinical investigations. While certain studies have reported a reduction in biomarkers of oxidative damage and an associated improvement in clinical outcomes, other trials have failed to demonstrate significant therapeutic benefit. These inconsistencies may stem from a variety of factors, including heterogeneity in study design, differences in antioxidant dosing regimens, variability in bioavailability, and the specific stage or severity of disease in the patient populations studied [61].
Polyphenols, a diverse group of phytochemicals abundantly present in fruits, vegetables, tea, and red wine, have also been recognized for their potent antioxidant and anti-inflammatory properties. Both in vitro and in vivo studies have provided compelling evidence that polyphenols exert protective effects by neutralizing reactive oxygen species, modulating signaling pathways involved in inflammation, and preserving cellular redox homeostasis. Among these, epigallocatechin gallate (EGCG), the principal catechin in green tea, has emerged as a particularly promising compound. Experimental and clinical data have demonstrated that EGCG is capable of reducing ROS levels, inhibiting tumor proliferation, and improving parameters of cardiovascular health, underscoring its potential utility across multiple disease contexts [62,63].
In addition to naturally occurring compounds, several synthetic and endogenous antioxidants have shown clinical promise. Agents such as N-acetylcysteine, alpha-lipoic acid, and coenzyme Q10 have been investigated for their ability to modulate oxidative stress pathways and ameliorate disease-related complications. These compounds have demonstrated beneficial effects in the management of conditions such as type 2 diabetes mellitus, cardiovascular disease, and chronic obstructive pulmonary disease. Their mechanisms of action include replenishment of intracellular glutathione levels, scavenging of free radicals, and stabilization of mitochondrial function, which collectively contribute to a reduction in oxidative damage and an improvement in clinical outcomes [64].
Beyond pharmacological interventions, lifestyle modifications constitute a cornerstone of antioxidant-based strategies in chronic disease management. Regular physical activity has been shown to upregulate endogenous antioxidant enzymes, such as superoxide dismutase and glutathione peroxidase, thereby enhancing the body’s intrinsic defense mechanisms against oxidative stress. Similarly, smoking cessation leads to a measurable decrease in systemic oxidative burden and an associated improvement in pulmonary and cardiovascular function. Nutritional interventions, particularly adherence to a Mediterranean diet rich in fruits, vegetables, whole grains, legumes, nuts, and olive oil, have been linked to improved antioxidant capacity and reduced incidence of chronic diseases. Notably, evidence from randomized controlled trials indicates that such dietary patterns can significantly attenuate oxidative stress markers and decrease the risk of adverse cardiovascular events in high-risk populations [65,66].
Table 3 summarizes representative antioxidant therapies and lifestyle interventions evaluated in recent research [39–60]. It outlines the principal compounds, dietary models, and behavioral modifications shown to influence oxidative stress markers and clinical outcomes, as well as their limitations and methodological constraints. The collected evidence indicates that while antioxidant supplementation yields inconsistent clinical benefits, lifestyle-based approaches such as regular physical activity, smoking cessation, and adherence to Mediterranean-type diets demonstrate the most reliable and reproducible effects on oxidative stress reduction and overall disease prevention.
Table 3. Antioxidant therapies and lifestyle interventions in oxidative stress-related chronic diseases
| Category | Representative agents/interventions | Evidence | Limitations/challenges | References |
| Antioxidant supplements | Vitamin C, vitamin E, alpha-lipoic acid, coenzyme Q10 | Protective effects in preclinical and small clinical studies | Poor bioavailability, inconsistent RCT outcomes, lack of standardized biomarkers | [39–44] |
| Pharmacological agents | Edaravone, N-acetylcysteine, mitoquinone (MitoQ) | Demonstrated reduction of oxidative markers in some trials | Limited clinical translation, short-term efficacy, small sample sizes | [45–48] |
| Dietary patterns | Mediterranean diet, DASH diet, polyphenol-rich foods (flavonoids, resveratrol, curcumin) | Consistent reduction of oxidative stress and inflammation markers, improved clinical outcomes in metabolic and cardiovascular diseases | Long-term adherence, variability in dietary composition, confounding lifestyle factors | [49–53] |
| Physical activity | Aerobic and resistance training, regular moderate exercise | Enhances endogenous antioxidant defense, improves mitochondrial function | Individual variability, poor adherence in chronic disease patients | [54–57] |
| Smoking cessation | Abstinence from tobacco, counseling programs | Reduces systemic oxidative stress and improves vascular function | High relapse rates, requires sustained behavioral support | [58–60] |
Recent advances in elucidating the molecular mechanisms underlying oxidative stress have significantly contributed to the development of innovative therapeutic strategies aimed at mitigating its detrimental effects across a range of chronic and degenerative diseases. Among the most promising approaches are mitochondria-targeted antioxidants, such as MitoQ and SkQ1. These compounds have been specifically engineered to accumulate within mitochondria, where they exert their effects by selectively attenuating the production of reactive oxygen species at the source. This targeted action holds particular therapeutic potential in the context of cardiovascular diseases, neurodegenerative disorders, and various forms of cancer, where mitochondrial dysfunction and oxidative damage are central to disease pathogenesis [67].
Another promising area of investigation involves gene therapy strategies designed to enhance endogenous antioxidant defenses. For instance, the overexpression of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) has demonstrated encouraging results in preclinical models of several oxidative stress-related conditions, including diabetes mellitus, neurodegenerative diseases, and chronic obstructive pulmonary disease. By restoring or augmenting the expression of these enzymes, it may be possible to rebalance redox homeostasis and attenuate disease progression [68].
In parallel, the field of nanomedicine has opened new avenues for improving the pharmacokinetic and pharmacodynamic properties of antioxidant compounds. Nanotechnology-based delivery systems such as liposomes and polymeric or lipid-based nanoparticles are actively being explored as means to enhance the bioavailability, tissue specificity, and controlled release of therapeutic antioxidants. Particularly in neurodegenerative disorders, where crossing the blood-brain barrier presents a significant therapeutic challenge, nanoparticle-based delivery systems offer the potential to significantly improve central nervous system penetration and target engagement [69].
Furthermore, small-molecule modulators of endogenous antioxidant pathways have garnered considerable interest. In particular, activators of the nuclear factor erythroid 2–related factor 2 (Nrf2) signaling pathway which serves as a master regulator of antioxidant gene expression have demonstrated potential in preclinical models. Activation of the Nrf2 pathway enhances the transcription of a range of cytoprotective genes involved in oxidative stress defense, including those encoding for glutathione biosynthesis, detoxification enzymes, and antioxidant proteins. By stimulating this intrinsic protective mechanism, Nrf2 activators may represent a versatile therapeutic modality applicable to a broad spectrum of oxidative stress–mediated pathologies [70].
While these emerging strategies are supported by encouraging preclinical data, it is important to emphasize that their clinical utility remains to be fully validated. Rigorous human studies are needed to establish the safety, efficacy, and long-term outcomes of these novel interventions before they can be integrated into routine clinical practice.
The analysis of the selected literature highlights oxidative stress as a central but heterogeneous mechanism underlying chronic diseases [1, 3, 4]. Although reactive oxygen species are consistently linked to inflammation, mitochondrial dysfunction, and macromolecular damage, the degree and nature of their contribution differ among disease groups. In cardiovascular disease and type 2 diabetes the evidence from experimental and clinical studies is comparatively strong, with consistent associations between oxidative stress markers and disease outcomes [10, 11, 12, 13, 17, 18, 19, 20, 21]. In neurodegenerative disorders and cancer the data are more fragmented, with mechanistic plausibility supported by experimental findings but weaker clinical translation [27, 28, 29, 30, 31, 33, 34, 35, 45, 46, 47, 49]. A key controversy in the field concerns the role of antioxidant supplementation. Numerous preclinical studies report protective effects of vitamin C, vitamin E, and alpha lipoic acid, yet randomized trials frequently fail to reproduce these benefits in patients [6, 7, 22, 23, 24]. Systematic reviews including Cochrane analyses emphasize that routine clinical use of antioxidant supplements cannot be recommended at present, though specific subgroups may benefit [26, 55]. The heterogeneity of results is attributed to variability in bioavailability, disease stage, and methodological limitations [6, 7]. Another important limitation is methodological variability. Many studies use small cohorts, surrogate biomarkers, and short follow up, while standardized assays and validated clinical endpoints are lacking, which complicates cross study comparison and weakens recommendations [6, 63]. Emerging therapeutic concepts including mitochondria targeted antioxidants, gene therapy approaches, and nanotechnology based delivery remain promising but are at early stages with limited robust human data [59, 60, 61, 62]. Lifestyle interventions show the most reliable benefit. Mediterranean style dietary patterns, smoking cessation, and regular exercise improve endogenous antioxidant defenses and reduce systemic inflammation, with supportive evidence from randomized and guideline level sources [17, 44, 57, 58]. Nonetheless, gaps persist regarding long term adherence, interactions with pharmacotherapy, and the contribution of specific dietary components [57, 58].
Oxidative stress is a unifying pathogenic factor in cardiovascular, metabolic, neurodegenerative, pulmonary, and oncological diseases. The available evidence consistently demonstrates its central role in promoting inflammation, mitochondrial dysfunction, and molecular damage.
However, despite extensive preclinical research, antioxidant therapies have not produced consistent or reproducible clinical benefits. Their translation into practice remains limited by poor bioavailability, methodological flaws, and contradictory outcomes. At present, lifestyle interventions such as dietary modification, physical activity, and smoking cessation constitute the only evidence-based means of reducing oxidative stress in patients.
Future research must move beyond descriptive associations and focus on validated biomarkers, rigorous long-term trials, and precisely targeted therapeutic strategies. Only through such approaches can oxidative stress modulation be established as a clinically relevant and effective tool in the management of chronic diseases.
Conceptualization: H. Knapik.
Methodology: P. Misiewicz, J. Witek, K. Janik.
Investigation and data collection: J. Rafalski, A. Klukowska, K. Barszcz.
Formal analysis: K. Zalisz, K. Janik.
Writing – original draft: M. Zając.
Writing – review and editing: P. Koszuta.
Supervision: H. Knapik.
All authors read and approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Artificial intelligence was used to assist in editing and formatting the manuscript, specifically for grammar checks and reference formatting. This is disclosed in accordance with journal guidelines.