- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 4. DOI 10.35630/2025/15/4.029
Background: Legg-Calvé-Perthes disease remains a significant challenge in pediatric orthopedics due to its unclear etiology, variable prognosis, and long-term risk of disabling hip deformities. Despite over a century since its first description, no consensus exists on optimal management. Recent studies have addressed epidemiology, genetics, imaging, and treatment, but evidence remains fragmented and sometimes contradictory.
Objective: The aim of this review was to systematically evaluate the current state of scientific knowledge on Legg-Calvé-Perthes disease. The analysis covers epidemiology, pathogenesis, clinical presentation, diagnostic approaches, and classification systems. In addition, the review examines potential risk factors and possible causes of the disease, which remain incompletely defined despite extensive research. Particular attention is given to treatment strategies, highlighting the inconsistencies and lack of uniformity in current clinical practice.
Methods: A comprehensive literature search was conducted in PubMed, Google Scholar, and Scopus, covering publications from January 2015 to April 2025. The review included original articles, systematic reviews, and meta-analyses, with inclusion criteria based on reliable research methodology, taking into account factors such as the characteristics of the study population, data collection methods, and approaches to data analysis. Publications that did not meet these criteria, were considered unreliable, or were based on outdated medical data were excluded from the review. A total of 50 articles were included.
Results: Incidence varies across populations, and etiology is multifactorial, involving vascular, genetic, and hormonal mechanisms. Treatment approaches are inconsistent, with conservative management favored in younger children and surgical interventions in older or advanced cases.
Conclusions: Legg-Calvé-Perthes disease remains a condition with an incompletely understood etiology, involving a multifactorial pathogenesis that includes genetic, environmental, metabolic, and hormonal factors. The complexity of LCPD's potential causes underscores the need for continued molecular, genetic, and clinical research, which could help to clarify the pathomechanisms of this condition. Treatment strategies remain heterogeneous and depend on patient age, disease stage, and institutional experience, with no consensus regarding the optimal timing or type of intervention. High-quality multicenter studies, including randomized controlled trials, are needed to reduce heterogeneity in clinical practice and to develop evidence-based recommendations.
Keywords: ‘Legg-Calvé-Perthes Disease’, ‘LCPD’, ‘Perthes’, ‘osteonecrosis’, ‘Avascular
necrosis’, ‘pediatric orthopedics’, ‘orthopaedic surgery’, ‘radiological outcomes’.
Legg-Calvé-Perthes disease (LCPD) is an idiopathic avascular necrosis of the femoral head and remains a significant clinical problem in pediatric orthopedics. It most commonly affects children and adolescents, with an incidence ranging from 0.4 to 29.0 per 100,000 under the age of 15, and a peak occurrence between 4 and 8 years of age. Although first described more than a century ago, its exact etiology remains unclear and is believed to be multifactorial, involving environmental, metabolic, and genetic factors [1, 2].
The disease is associated with variable prognosis and a long-term risk of hip deformities leading to disability, which underlines its clinical and social importance. Despite decades of research, there is still no consensus on the optimal treatment strategy, and therapeutic approaches differ widely across centers and countries, making it a subject of ongoing debate and clinical uncertainty.
Given these challenges, the significance of the topic lies not only in its impact on affected children and their quality of life but also in the need for unified, evidence-based guidelines to support clinical decision-making. In recent years, advances in epidemiology, genetic studies, imaging modalities, and treatment options have shed new light on LCPD, yet the findings remain fragmented and sometimes contradictory. This review addresses that gap by consolidating and critically analyzing the most recent developments. Its novelty lies in providing an up-to-date synthesis of current knowledge, with special emphasis on early diagnostic tools, the role of modern imaging, and the development of individualized treatment strategies. Unlike earlier publications, this article highlights the integration of new research trends and offers a forward-looking perspective on optimizing care for patients with LCPD.
The aim of this review was to systematically evaluate the current state of scientific knowledge on Legg-Calvé-Perthes disease. The analysis covers epidemiology, pathogenesis, clinical presentation, diagnostic approaches, and classification systems. In addition, the review examines potential risk factors and possible causes of the disease, which remain incompletely defined despite extensive research. Particular attention is given to treatment strategies, highlighting the inconsistencies and lack of uniformity in current clinical practice.
A comprehensive literature search was conducted in the PubMed, Google Scholar, and Scopus databases, covering publications from January 2015 to April 2025. The article was developed based on current scientific research, the latest reports, and existing expert guidelines. The review included original articles, systematic reviews, and meta-analyses, with inclusion criteria based on reliable research methodology, taking into account factors such as the characteristics of the study population, data collection methods, and approaches to data analysis. This approach ensures a reliable presentation of the topic within the context of contemporary medical knowledge, which is essential for the accuracy and credibility of the content. Publications that did not meet these criteria, were considered unreliable, or were based on outdated medical data were excluded from the review. Articles were identified using carefully selected keywords: “Legg-Calvé-Perthes Disease,” “LCPD,” “Perthes,” “osteonecrosis,” “Avascular necrosis,” “ pediatric orthopedics,” “orthopaedic surgery,” and “radiological outcomes.” . In total, 50 articles were included in the final analysis.
LCPD most commonly occurs in children between the ages of 2 and 14, with peak incidence around 5 or 6 years of age. There is a significant gender disparity—boys are affected much more frequently than girls, at a ratio of approximately 5:1. LCPD is most often diagnosed in children of Northern European descent, with incidence in the white population ranging widely from 1 in 400 to 1 in 35,000 children [3]. The regions with the highest incidence include the Faroe Islands, the British Isles, and Denmark. In other racial populations, the disease occurs only sporadically. Notable variability in incidence has been observed between different countries and regions, as well as among various ethnic groups [4]. There is also a trend toward higher prevalence in populations with lower socio-economic status. Perthes disease is more frequently observed in families with lower levels of education; however, no significant differences have been noted in relation to income [5]. Pathological changes occur with similar frequency in both the right and left hip joints. The vast majority of cases are unilateral—observed in approximately 93.3% of patients. Bilateral involvement is much less common, occurring in only about 6.7% of cases [6].
Table 1. Incidence of Legg-Calvé-Perthes Disease by Region (1964–2006) [4].
| Region | Range of Incidence (%) |
| Asia | 0.4–4.5 |
| Africa | 0.45–10.8 |
| North America | 5.1–5.7 |
| British Isles | 5.5–15.6 |
| Scandinavia | 8.0–29.0 |
Table 2. Incidence of Legg-Calvé-Perthes Disease by Skin Color / Ethnicity (1964–2006) [4].
| Skin color / Ethnicity | Range of Incidence (%) |
| White | 5.1 – 29.0 |
| Indo-Med / Indo-Malay | 0.4 – 4.5 |
| Black | 0.45 – 1.8 |
| Mixed | 1.73 |
Despite years of research and sustained interest in Legg-Calvé-Perthes disease, its exact etiology remains incompletely understood. It is currently believed that the disease has a multifactorial origin, with environmental and metabolic factors playing a significant role in its pathogenesis. Additionally, cases of familial occurrence of LCPD have led to the hypothesis of a possible genetic basis for the disease. Human blood is a complex mixture of substances, and disturbances in its composition may significantly influence the development of Legg-Calvé-Perthes disease. Scientific literature suggests a potential link between this condition and hemostatic disorders. Among the most commonly reported abnormalities are the G20210A prothrombin mutation, deficiencies of protein C and protein S, elevated levels of factor VIII, and the presence of factor V Leiden. These types of disorders, collectively referred to as thrombophilia, may predispose individuals to venous thrombosis. Such thrombosis, by obstructing venous outflow from the femoral head epiphysis, can result in local ischemia and necrosis. However, it is important to note that thrombosis in childhood is relatively rare, and no clear-cut cases of coagulation disorders coexisting with LCPD in pediatric patients have been documented to date. Nevertheless, it is possible that Legg-Calvé-Perthes disease may represent an as-yet unidentified hematological disorder, in which an undetected mutation may affect coagulation mechanisms—though its exact pathophysiology remains unclear [7, 8, 21]. In the search for the causes of ischemia in the femoral head, attention should be directed not only toward the properties of blood but also toward the characteristics of blood vessels. These conclusions have prompted further molecular studies on markers involved in endothelial cell dysfunction. These include markers of angiogenesis, inflammation, and apoptosis. Promising research is also being conducted on genes involved in bone turnover [9]. The literature suggests a potential association between LCPD and a polymorphism in the gene encoding endothelial nitric oxide synthase (eNOS), which is linked to reduced nitric oxide levels in plasma. Nitric oxide (NO), alongside various growth factors, plays a key role in the process of new blood vessel formation, known as angiogenesis. As a signaling molecule, nitric oxide is involved in regulating vasodilation, blood flow, and the proliferation and migration of endothelial cells. These processes are essential for the proper development and remodeling of the vascular network. In the context of Legg-Calvé-Perthes disease, impaired angiogenesis may play a significant role in the pathogenesis of disrupted blood supply to the femoral head [10, 11]. Additionally, the role of mutations in the type II collagen gene (COL2A1) is being considered. Such mutations may be responsible for skeletal dysplasia syndromes and could potentially predispose individuals to the development of Perthes disease [12–14]. One of the more intriguing hypotheses regarding the etiology of LCPD is its association with hormonal disorders, particularly pituitary insufficiency. This clinical syndrome is characterized by a partial or complete deficiency of one or more hormones produced by the pituitary gland, which can lead to various metabolic and developmental complications, especially in childhood. Cases have been reported in the literature where patients with LCPD exhibited growth hormone (GH) deficiency and decreased levels of insulin-like growth factor type 1 (IGF-1), a key mediator of GH action. Notably, IGF-1 levels in children with LCPD are significantly lower compared to those observed in the general population, suggesting a potential role of disruptions in the GH–IGF-1 axis in the pathogenesis of this condition. In one published case study, a patient diagnosed with multiple pituitary hormone deficiency (MPHD) was also confirmed to have LCPD, further supporting the possible link between pituitary insufficiency and avascular necrosis of the femoral head. Therefore, it is recommended that children with diagnosed growth hormone deficiency be carefully monitored for potential symptoms of LCPD, both before and during GH therapy, which may be crucial for early detection and treatment of the disease [15, 16].
Exposure to tobacco smoke is cited as one of the potential risk factors for the development of Perthes disease, although the pathogenic mechanisms remain not fully understood. Results from some studies suggest that in utero exposure to this factor may significantly increase the likelihood of developing the condition [17]. There is also evidence suggesting a link between Legg-Calvé-Perthes disease and attention deficit hyperactivity disorder (ADHD). Patients with a history of LCPD have been found to have a statistically significantly higher risk of developing ADHD compared to individuals of the same age and sex without LCPD. This may indicate the presence of shared risk factors in the development of both conditions [18]. The heritability of ADHD is estimated at 77–88%, indicating that genetic factors play a major role in its pathogenesis. However, this disease is not the result of a single gene but rather a complex interaction of multiple genes. Additionally, gene-environment interactions play a significant role. Environmental factors that may potentially contribute to the development of ADHD include maternal smoking and alcohol consumption during pregnancy, low birth weight, premature births, and exposure to environmental toxins such as organophosphate pesticides, polychlorinated biphenyls, and zinc. However, there is a lack of studies directly comparing the impact of ADHD risk factors with the pathogenesis of Perthes disease. Such analyses could represent a promising step toward a better understanding of the etiology of LCPD [19, 20].
Symptoms. The onset of Perthes disease is often mild and non-specific, which can lead to delayed diagnosis. One of the earliest symptoms is limping, accompanied by a limited range of motion in the hip joint—particularly in abduction and internal and external rotation. Patients may experience pain around the hip area, which can radiate toward the knee. These symptoms most often worsen after physical exertion. In more advanced stages of the disease, muscle atrophy of the thigh and gluteal muscles occurs due to the child’s reduced physical activity. This may lead to a positive Trendelenburg sign—pelvic drop on the opposite side when standing on one leg. Another possible symptom during the course of Perthes disease is morning stiffness of the hip joint [22–25].
Imaging Diagnostics. The most commonly used imaging method for diagnosing Perthes disease is conventional X-ray imaging performed in two projections: anteroposterior (AP) and lateral. Obtaining images in two planes significantly reduces the risk of diagnostic errors and enables accurate diagnosis. X-ray is considered the gold standard for identifying LCPD. However, it is important to emphasize that conventional X-rays do not provide detailed information needed to assess the structures of the hip joint components. Therefore, magnetic resonance imaging (MRI) is used to evaluate the disease’s progression and to aid in selecting the appropriate treatment method. Another diagnostic tool helpful in LCPD assessment is ultrasonography (USG). The advantages of ultrasound include its wide availability, low cost, and lack of side effects. Additionally, it is a quick and non-invasive examination. This is especially important in young children, for whom diagnostic procedures can be challenging due to limited ability to cooperate and a lack of understanding about the process and purpose of medical examinations. Compared to magnetic resonance imaging, which is time-consuming and requires the patient to remain still, ultrasound usually does not require sedation in young children. Ultrasonography appears to be a good complementary method for evaluating patients with LCPD; however, it should not be used as the sole imaging technique because it is subjective and only visualizes soft tissues. Studies have shown that ultrasound is useful in monitoring joint effusion, assessing femoral head extrusion, and recognizing the healing phase. Computed tomography (CT) has also been applied in LCPD diagnosis, as it depicts areas of osteosclerosis surrounding the necrotic bone and reveals subchondral bone fractures. CT is also used in surgical treatment planning through 3D imaging, allowing detailed three-dimensional visualization of anatomical structures, which enables precise surgical intervention planning and minimizes the risk of complications [2, 26–28]. Another supportive diagnostic method is arthrography — a procedure involving the injection of contrast material into the joint cavity, followed by imaging using one of the aforementioned techniques. This examination allows dynamic assessment of joint compliance in various positions. It is particularly useful in advanced cases with flattening or collapse of the femoral head. In early stages, the arthrographic image may remain normal. Arthrography serves as a valuable tool for assessing disease progression and planning further therapeutic management [33].
![Fig. 1 Preoperative radiograph showing morphological sequelae of Legg-Calvé-Perthes disease in the left hip - oversized ellipsoid femoral head, GT elevation, shortened femoral neck, and mild acetabular dysplasia [49].](artikel-29-bild-1.jpg)
Fig. 1 Preoperative radiograph showing morphological sequelae of Legg-Calvé-Perthes disease in the left hip - oversized ellipsoid femoral head, GT elevation, shortened femoral neck, and mild acetabular dysplasia [49].
Classification Systems. An important aspect of diagnosing Legg-Calvé-Perthes disease is the proper classification of pathological changes. This is crucial for further clinical management, as it influences the choice of the appropriate therapeutic strategy—both surgical and conservative methods—and also plays a significant role in prognosis assessment. Over a century of clinical experience since the first reports of LCPD has enabled the development of several classification systems that facilitate the assignment of different stages of the disease to the corresponding categories.
Herring Classification. Currently the most commonly used classification in Perthes disease. It is based on the assessment of a single criterion on the X-ray image—the height reduction of the lateral pillar of the femoral head:
A – height of the lateral pillar fully preserved.
B – height of the lateral pillar preserved between 50% and 100%.
C – height of the lateral pillar preserved below 50% [29].
![Fig. 2 Herring classification [29].](artikel-29-bild-2.jpg)
Fig. 2 Herring classification [29].
Modified Waldenström Classification. This is a modernized scale based on Waldenström’s 1922 classification. It involves radiological analysis of the disease’s progression and helps identify the onset of epiphyseal, metaphyseal, and acetabular events associated with worsening prognosis:
IA – sclerotic phase without loss of epiphyseal height.
IB – sclerotic phase with loss of epiphyseal height.
IIA – epiphyseal fragmentation – radiologically visible one or two vertical cracks.
IIB – advanced fragmentation without lateral displacement of the bone.
IIIA – formation of new bone tissue in the peripheral necrotic area without normal texture – involving less than one-third of the epiphyseal area.
IIIB – new bone texture is normal and involves more than one-third of the epiphyseal area.
IV – femoral head assumes a stable shape, which may be normal or show signs of flattening and deformation [30].
Catterall Classification. This commonly used classification is based on the analysis of radiographic images, taking into account the extent of necrosis affecting the femoral head:
Group I – necrosis limited to the anterosuperior part of the epiphysis, not exceeding 25% of the surface.
Group II – necrosis limited to the anterosuperior part of the epiphysis, not exceeding 50% of the surface.
Group III – necrosis limited to 75% of the epiphysis.
Group IV – the entire epiphysis undergoes necrosis and fragmentation [31, 32].
An important issue remains the selection of the optimal therapeutic strategy. Despite many years of clinical experience and research on Legg-Calvé-Perthes disease, there are still no strictly defined criteria that would clearly determine the treatment approach. Treatment can be divided into conservative and surgical methods. Conservative management is primarily recommended for children under 6 years of age, while surgical interventions are reserved for older children, severe cases, or situations where non-operative treatment does not yield the expected results. The main goal of both types of treatment is to preserve the femoral head by limiting the mechanical load on the hip joint, which leads to its deformation. Regardless of the treatment method, there is strong evidence that early intervention provides the most favorable outcomes [34].
Conservative Treatment. Although conservative treatment is widely used in cases of Legg-Calvé-Perthes disease (LCPD), there is no consensus on how it should be implemented, which patients are suitable candidates, or at what stage of treatment it should be initiated. Studies have shown that children up to eight years of age demonstrate better outcomes with conservative management compared to older patients. This phenomenon is likely due to the longer time available for remodeling of the femoral head before skeletal maturity—this time is longer in younger patients. Additionally, older children typically have greater body mass, which increases the mechanical load on the hip joint and promotes the development of irreversible femoral head deformities. Conservative treatment of Perthes disease includes a variety of complementary therapeutic approaches, such as pain-relieving and anti-inflammatory pharmacotherapy, individually tailored physical activity and physiotherapy, patient and caregiver education, and psychological support. Each of these components plays a crucial role in a comprehensive therapeutic approach, and the integration of different forms of treatment yields the best outcomes. Research suggests that the use of nonsteroidal anti-inflammatory drugs (NSAIDs), such as acetylsalicylic acid (ASA) and ibuprofen, may significantly influence the course of Perthes disease. Their anti-inflammatory action helps reduce the inflammatory response of the synovial membrane in the hip joint, potentially slowing the progression of pathological changes. Additionally, the analgesic properties of these medications are beneficial in alleviating clinical symptoms, particularly during disease flare-ups. Of particular interest is the antiplatelet effect of acetylsalicylic acid, which results from its inhibition of cyclooxygenase (COX) and, consequently, the suppression of platelet aggregation. There are hypotheses suggesting that ASA may improve microcirculation within the femoral head, potentially supporting the revascularization of necrotic tissue. However, the reliability of this evidence is currently low, and further research is needed to validate this theory. It is also essential to note that the use of acetylsalicylic acid in children should be conducted only under strict medical supervision. Improper use of this drug in pediatric patients carries a risk of Reye’s syndrome a rare and potentially fatal childhood condition characterized by acute non-inflammatory encephalopathy and fatty liver failure [35, 36, 37]. An individualized physiotherapy program is an extremely important component of treatment. Studies have shown that physiotherapy improves the range of motion in the hip joint, preventing stiffness and contractures. Maintaining the activity of the thigh and gluteal muscles through targeted exercises helps reduce the risk of muscle atrophy and prevents complications such as a positive Trendelenburg sign. However, it is important to emphasize that not all forms of physical activity are recommended, nor are they appropriate at every stage of the disease. Weight-bearing activities that exert significant force on the affected limb are contraindicated at all stages of Perthes disease, as excessive mechanical stress on the femoral head may lead to secondary deformation. In the early phase of the disease, low-load strengthening exercises are recommended 2–3 times per week. In contrast, stretching exercises and low-impact activities such as swimming, cycling, short walks, or horseback riding are beneficial at all stages of the disease and can generally be performed without restrictions. Studies indicate that stretching exercises should be performed at least once daily. Nevertheless, there is no clear consensus on the optimal schedule for resuming full physical activity. An important adjunct to physical therapy is the use of appropriate orthopedic devices. Orthoses, plaster casts, and specialized orthopedic braces help maintain the hip joint in a position of abduction and internal rotation, which promotes centralization of the femoral head within the acetabulum. This positioning is considered the most favorable for the remodeling process, as it minimizes the risk of deformation. Additionally, some orthoses allow for partial unloading of the hip during walking, which is particularly important during periods of pain exacerbation. In everyday functioning, mobility aids such as elbow crutches, walkers, and wheelchairs can also be helpful in supporting the child’s mobility. One of the key aspects of effective treatment for Perthes disease is proper education of both the parents and the child. Awareness of the importance of regular physiotherapy, avoiding excessive mechanical loads, and adhering to medical recommendations has a real impact on the course of the disease. One of the key aspects of effective treatment for Perthes disease is proper education of both the parents and the child. Awareness of the importance of regular physiotherapy, avoiding excessive mechanical loads, and adhering to medical recommendations has a real impact on the course of the disease. Educating the child presents a particular challenge—young patients often do not fully understand the reasons behind the restrictions imposed by the condition. It is essential that the child understands the importance of following recommendations, including avoiding inappropriate forms of physical activity. Children should also be taught to recognize warning signs, such as pain, and to respond appropriately by immediately ceasing the activity in question.
The emotional aspect must not be overlooked. For many children, the treatment period can be mentally challenging. Isolation from peers during physical activities, having to give up favorite games, or wearing visible assistive devices may lead to frustration, feelings of rejection, or decreased self-esteem. Therefore, emotional support should be an integral part of care. This includes age-appropriate communication and, if needed, psychological consultation. For these reasons, the assessment of the patient’s mental health should be a core element of treatment planning from the early stages. Mobility aids such as crutches, wheelchairs, or walkers should be introduced thoughtfully and only when truly necessary. During childhood and adolescence, peer acceptance plays a significant role, and the use of visible assistive devices can negatively affect a child’s mood and self-image [36, 38, 39].
Surgical Treatment. Surgical management of Legg-Calvé-Perthes disease (LCPD) is varied and primarily aims to maintain the femoral head within the acetabulum. The most commonly used procedures include femoral osteotomies, pelvic osteotomies, and combined surgical techniques. Surgery is typically indicated in children over the age of 8, especially in cases where conservative treatment fails to produce the desired outcomes or when the prognosis is poor. As with conservative therapy, there are no standardized criteria regarding the choice of surgical method or the optimal timing for its implementation. It is important to note that each surgical technique has its own advantages and limitations, and carries a risk of complications [40].
One of the most commonly used surgical techniques in the treatment of LCPD is Femoral Varus Osteotomy (FVO). The goal of this procedure is to centralize the femoral head within the acetabulum, which promotes proper remodeling of its structure and can alter the natural course of the disease—particularly during the fragmentation stage—by slowing its progression to more advanced stages [41]. This technique was first described by Axer in 1963 and has since undergone numerous modifications. Today, the osteotomy is most commonly performed through an intertrochanteric approach, and advancements in stabilization techniques have allowed the replacement of traditional plates with more advanced implants featuring a predefined varus angle. The surgical method can be tailored based on the extent of necrosis affecting the femoral head. The operation offers several options for optimally positioning the femoral head within the acetabulum. Technical variations include correction in the frontal plane (varus angulation), sagittal plane (flexion-extension osteotomy), and transverse plane (derotational osteotomy), enabling precise alignment of the femoral head in the socket. These practices allow for an individualized approach based on patient anatomy. It is worth noting that this procedure is technically simpler to perform than pelvic osteotomies, which may influence the surgeon’s choice. However, FVO also has significant drawbacks. The most common complication is limb shortening on the operated side. Literature reports a typical discrepancy of approximately 1 cm, which may lead to gait disturbances. Furthermore, the surgery causes proximal displacement of the gluteus medius and minimus muscle attachments, potentially reducing muscle strength and contributing to the development of Trendelenburg gait and Duchenne’s intermittent claudication. For this reason, comprehensive postoperative physiotherapy is a crucial component of treatment. This includes the use of shoe lifts to equalize limb length, strengthening exercises for the gluteal muscle group, and maintenance of proper hip joint mobility [42, 43].
![Fig. 3 Femoral varus osteotomy (FVO) [40].](artikel-29-bild-3.jpg)
Fig. 3 Femoral varus osteotomy (FVO) [40].
The Salter Innominate Osteotomy (SIO) involves a transverse osteotomy performed proximal to the hip joint. The distal fragment of the osteotomy is then displaced laterally and anteriorly. This maneuver is intended to improve the coverage of the femoral head by the acetabulum. In the next stage of the procedure, a bone wedge is harvested from the iliac crest and used to maintain the corrected position of the osteotomy. The final phase of the surgery involves the fixation of all bony elements to ensure optimal stabilization of the anatomical structures. Precise immobilization of the bone fragments is crucial to create proper conditions for bone union and tissue regeneration. The most commonly used tools for fixation are Kirschner wires. However, their use may be associated with complications such as migration of the wires beyond the drill channel or into the pelvic cavity. This poses a risk to surrounding structures and often necessitates surgical removal of the wires. Additionally, if displacement occurs shortly after the procedure, it may lead to pelvic instability, which significantly increases the risk of serious complications. An interesting alternative to traditional fixation methods is the use of resorbable screws. Their primary advantage is that implant removal is not required after the healing process is complete, eliminating the need for a second surgery. As a result, the risks associated with general anesthesia are reduced, hospital stay is shortened, and overall treatment costs are lowered. Importantly, the absence of the need to remove the implants allows the surgeon to position them more optimally. Furthermore, resorbable screws are associated with a lower risk of infection compared to Kirschner wires. Unfortunately, according to available studies, resorbable screws demonstrate lower mechanical stability than traditional wires, which can significantly affect their application in certain clinical scenarios [44, 45].
Triple Pelvic Osteotomy involves cutting all three bones that form the pelvis — the ilium, pubis, and ischium — to reorient the acetabulum of the hip joint. The osteotomies are performed as close to the joint as possible, which promotes effective healing and provides high postoperative stability. Unlike procedures performed on the femur, triple osteotomy does not disrupt the biomechanics of the hip joint. For instance, a potential undesirable consequence of a femoral varus osteotomy is weakening of the hip abductor muscles — a complication not observed with triple pelvic osteotomy. Notably, this procedure also does not result in limb shortening. However, the method has limitations. It involves a technically demanding surgical technique and carries a risk of significant blood loss [46]. In severe cases, such as advanced femoral head deformity where neither femoral nor pelvic procedures alone are likely to restore normal hip joint biomechanics, combined surgical interventions are recommended. These procedures are most commonly performed in older patients with prominent clinical symptoms and yield the best outcomes in terms of restoring the physiological function of the hip joint. Nevertheless, combining two surgical techniques also increases the risk of complications specific to each individual method, as well as those arising from their simultaneous use. Moreover, the use of two techniques prolongs the duration of surgery. Studies have shown that longer surgical times are associated with a higher risk of postoperative wound infection [47, 48].
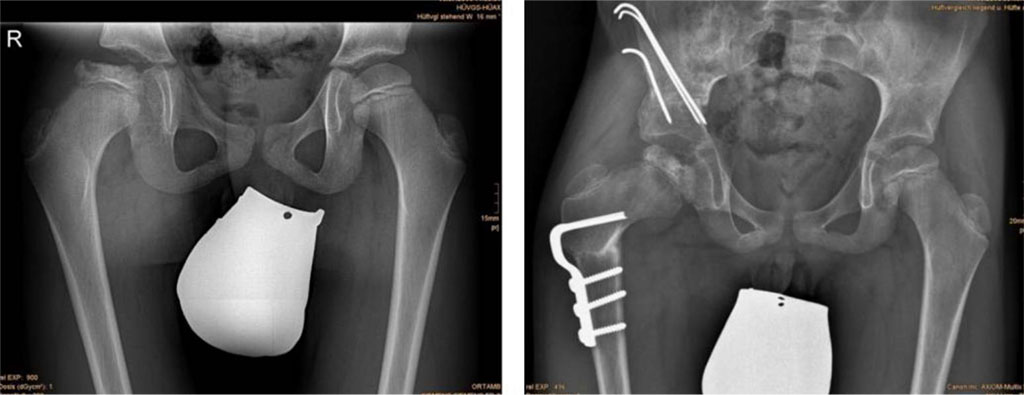
Fig. 4 (left) Anteroposterior radiograph of a nine-year-old male with LCPD; (right) Result three months postoperatively after combined femoral and iliac osteotomy [50].
Legg-Calvé-Perthes Disease (LCPD) is an idiopathic avascular necrosis of the femoral head, most commonly affecting children between the ages of 2 and 14, with the peak incidence occurring between 4 and 8 years of age. It is estimated that boys are affected five times more frequently than girls. In the majority of cases (93.3%), the disease involves only one hip joint. The prevalence of LCPD varies significantly across geographic and ethnic groups, with the highest incidence observed in Northern European populations [2, 4]. Despite many years of research and growing interest in LCPD, its etiology remains not fully understood. Currently, it is accepted that the ischemia of the femoral head is multifactorial, involving environmental, metabolic, and genetic factors. Increasing evidence points to the role of vascular abnormalities and endothelial dysfunction in the development of LCPD, particularly in the context of reduced nitric oxide bioavailability due to eNOS gene polymorphisms. Mutations in the COL2A1 gene, associated with skeletal dysplasia, may also contribute to the disease. One hypothesis links LCPD to growth hormone deficiency and low IGF-1 levels, especially in children with pituitary insufficiency. There is also growing evidence of a potential association between the disease and prenatal exposure to tobacco smoke, as well as comorbidity with ADHD, which may reflect shared genetic and environmental determinants. The complexity of LCPD's potential causes underscores the need for continued molecular, genetic, and clinical research, which could help to clarify the pathomechanisms of this condition and potentially improve early diagnosis and treatment strategies. The fragmentation of pathogenetic theories highlights the absence of a prevailing paradigm. Such inconsistencies stem not only from methodological heterogeneity but also from small sample sizes and ethnic or geographical variations. In clinical practice, these discrepancies pose significant challenges to the development of standardized diagnostic and therapeutic guidelines [10, 12, 15] .
The symptoms of Legg-Calvé-Perthes Disease (LCPD) often develop gradually and initially present mildly and non-specifically, which can lead to delays in diagnosis. One of the first and most characteristic symptoms is limping, which may appear suddenly or develop progressively. This is accompanied by restricted hip joint mobility, particularly in abduction and both internal and external rotation. Pain in the hip area is common, often radiating to the thigh or knee, and typically worsens after physical activity. As the disease progresses, a Trendelenburg sign may appear. Some patients also experience morning stiffness of the hip joint. The primary diagnostic method is X-ray imaging in anteroposterior (AP) and lateral projections; however, magnetic resonance imaging (MRI) allows for more accurate evaluation. Although less precise, ultrasound (US) is fast, inexpensive, and safe—making it particularly useful in younger children. Computed tomography (CT) and arthrography serve as supplementary tools, providing detailed anatomical information that aids in surgical planning [24, 33]
Accurate classification of pathological changes in LCPD is essential for guiding clinical decision-making, influencing the choice between surgical and non-surgical management, and allowing for reliable prognosis assessment. Several classification systems have been developed over the years, based on radiographic findings and clinical symptoms. The most commonly used systems include: the Herring classification, the modified Waldenström classification, and the Catterall classification [29].
Choosing the optimal therapeutic strategy in LCPD remains challenging due to the lack of standardized treatment guidelines. Management is typically divided into conservative approaches, recommended mainly for children under the age of six, and surgical methods, more often used in older children, in advanced disease stages, or when conservative treatment fails. The main goal of therapy—regardless of type—is to protect the femoral head from deformation by minimizing stress on the hip joint. Early intervention significantly improves treatment outcomes.
Conservative treatment includes pharmacotherapy, physiotherapy, appropriate physical activity, orthopedic aids, patient and family education, and emotional support. Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and acetylsalicylic acid (ASA), may help slow disease progression by reducing inflammation. ASA might also improve microcirculation, although supporting evidence is limited. Physiotherapy focuses on preserving joint mobility and strengthening or stretching key muscle groups to delay or prevent deformities. Load management is critical—strenuous activities should be avoided in favor of low-impact exercises such as swimming or cycling. Orthotic devices, plaster casts, and braces can help keep the femoral head in a centralized position conducive to proper remodeling. Education and psychological support for the patient and caregivers are as important as the physical treatment. Understanding the disease and the importance of compliance with treatment recommendations directly affects outcomes. Emotional difficulties, stemming from social isolation, mobility limitations, and the use of visible assistive devices, can negatively impact a child’s self-esteem. Therefore, psychological evaluation should accompany the treatment process from the beginning [34].
One of the most commonly used surgical techniques in LCPD is femoral varus osteotomy (FVO), aimed at centralizing the femoral head within the acetabulum and slowing disease progression. This procedure is technically simpler than pelvic osteotomies and can be modified according to individual anatomical conditions. However, it carries risks such as limb shortening and muscle weakness, making postoperative physiotherapy an essential part of recovery. Another method is the Salter innominate osteotomy (SIO), which improves femoral head coverage by redirecting part of the pelvis and stabilizing it—typically using Kirschner wires. However, these wires may migrate and lead to complications. An alternative is the use of absorbable screws, which do not require removal and reduce infection risk, though they are less stable than traditional wires. The triple pelvic osteotomy technique involves cutting all three pelvic bones to reorient the acetabulum without affecting joint biomechanics or limb length. However, it requires high surgical skill and carries a risk of significant blood loss. In severe or advanced cases, combined procedures on both the femur and pelvis are recommended. These offer the best chance of restoring normal hip joint function but also increase the risk of complications and prolong surgical time, which raises the likelihood of infection. There are numerous surgical techniques used to treat LCPD, varying in invasiveness, extent of intervention, and expected clinical outcomes. Each method has its strengths and limitations that must be considered during treatment planning. The choice of procedure should be individualized, taking into account the patient’s age, disease stage, clinical condition, and therapeutic goals. Equally important are the surgeon’s skills and experience, which can greatly influence the effectiveness of the chosen intervention. The available literature presents inconsistent findings regarding the effectiveness of different therapeutic strategies. Some studies indicate a superiority of surgical treatment, particularly in older patients and those in more advanced stages of the disease. Conversely, other reports suggest that conservative management in children under the age of six may yield comparable outcomes. The absence of randomized controlled trials (RCTs), however, means that most available conclusions are based on retrospective analyses and cohort studies, which substantially limits their evidentiary strength and hinders the formulation of definitive clinical recommendations [1, 40].
This review highlights that Legg-Calvé-Perthes disease (LCPD) remains a condition with an incompletely understood etiology, involving a multifactorial pathogenesis that includes genetic, environmental, metabolic, and hormonal factors. Epidemiological data confirm significant variability in disease incidence across populations, suggesting a substantial influence of both genetic and environmental determinants. The complexity of LCPD's potential causes underscores the need for continued molecular, genetic, and clinical research, which could help to clarify the pathomechanisms of this condition.
Despite advances in imaging techniques, particularly magnetic resonance imaging (MRI), LCPD diagnosis still relies predominantly on conventional radiographs. The lack of standardized imaging criteria and classification systems complicates clinical decision-making.
Treatment strategies for LCPD remain heterogeneous; clinical decisions depend on patient age, disease stage, and institutional experience, and consensus on the optimal timing and type of intervention has not been reached. The absence of randomized controlled trials limits the ability to formulate evidence-based recommendations.
There is a clear need for further high-quality multicenter studies, including prospective and randomized trials, to establish standardized diagnostic and therapeutic guidelines and to improve outcomes in children with LCPD.
Conceptualization: Konrad Kotte
Methodology: Konrad Kotte
Investigation: Konrad Kotte, Wojciech Pabis, Julia Lipiec
Formal analysis: Konrad Kotte, Julia Lipiec, Kamil Łebek
Resources: Konrad Kotte, Alicja Bury, Patryk Biesaga
Writing – original draft: Konrad Kotte, Daria Litworska-Sójka, Daniel Narożniak
Writing – review and editing: Konrad Kotte, Bartosz Komsta, Kordian Kotte
Visualization: Konrad Kotte, Kamil Łebek, Patryk Biesaga
Supervision: Konrad Kotte, Daria Litworska-Sójka, Bartosz Komsta
Project administration: Konrad Kotte, Kordian Kotte, Daniel Narożniak
All authors have read and agreed with the published version of the manuscript.
Artificial intelligence tools (e.g., ChatGPT, OpenAI) were used to assist with language editing, structural refinement, and the formulation of selected textual segments (e.g., background synthesis, objectives, conclusions). All AI-assisted content was critically reviewed, fact-checked, and finalized by the authors.
The study did not receive special funding.
The authors declare no conflict of interest.