- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 4. DOI 10.35630/2025/15/4.019
Aims: Gastroesophageal reflux disease (GERD) is a widespread condition affecting approximately 13 percent of the global population. One of its most common extraesophageal manifestations is chronic hoarseness. This review aims to explore the relationship between GERD and chronic hoarseness, focusing on epidemiology, pathophysiological mechanisms, diagnostic approaches, and treatment outcomes, and to test the hypothesis that the lack of standardized diagnostic criteria for laryngopharyngeal reflux (LPR) significantly influences reported prevalence rates and treatment results.
Methods: A comprehensive review of the literature up to 2025 was conducted to assess the association between GERD and chronic hoarseness. The analysis included data on prevalence, diagnostic methodologies such as 24 hour pH impedance monitoring, endoscopy, laryngoscopy, and esophageal manometry, and treatment modalities including pharmacologic, surgical, and behavioral interventions.
Results: The prevalence of chronic hoarseness among GERD patients can reach 97 percent, with professional voice users affected more often than the general population. Rates vary widely between studies due to differences in diagnostic criteria and assessment methods. Pathophysiological mechanisms include mucosal injury from gastric acid, inflammatory changes, and neural dysregulation linked to LPR. Diagnostic accuracy is limited by the absence of standardized LPR criteria. Proton pump inhibitors are effective in most erosive cases but less so in non-erosive disease, with up to 40 percent showing inadequate response. Potassium competitive acid blockers provide similar efficacy with improved pharmacokinetics. Adjunctive therapies such as prokinetics, H2 receptor antagonists, baclofen, alginates, and voice therapy can improve outcomes. Laparoscopic fundoplication offers long-term relief in selected patients, and less invasive endoscopic techniques are options for those unsuitable for surgery.
Conclusions: GERD-related hoarseness is a clinically significant condition that requires early recognition and targeted management. The hypothesis that a lack of standardized diagnostic criteria for LPR significantly influences prevalence estimates and treatment outcomes is supported by the reviewed evidence. Standardization of diagnostic protocols is essential to improve epidemiological accuracy and guide therapy. Optimal management requires a personalized approach that may combine pharmacological therapy, surgical intervention, and behavioral modifications, supported by multidisciplinary collaboration.
Keywords: gastroesophageal reflux disease, GERD, laryngopharyngeal reflux, LPR, chronic hoarseness, dysphonia, voice disorder, proton pump inhibitors, anti-reflux surgery, voice therapy
Gastrointestinal reflux disease (GERD) is a condition that involves the reflux of stomach contents, including pepsin, bile, acid, food material, and gastrointestinal microorganisms into the esophagus and other areas beyond it. This can result in troublesome symptoms not typically associated with the esophagus. Approximately 13% of the global population experiences GERD symptoms once a week or more, with higher prevalence observed in certain geographic regions [1]. Complications involving the esophagus include Barrett's esophagus, gastric cardia cancer, dysphagia, peptic strictures, and peptic ulcers. GERD may result in laryngopharyngeal reflux (LPR), in which gastric contents reach the larynx and pharynx, leading to symptoms such as chronic cough, globus sensation, and hoarseness [2]. Although LPR is increasingly viewed as a distinct condition, it shares considerable overlap with GERD in terms of pathophysiology and symptomatology. The reported prevalence of laryngopharyngeal reflux (LPR) varies substantially between studies and across countries, primarily because of differences in diagnostic criteria and assessment methods. This heterogeneity underscores the absence of standardized methodology and its considerable impact on the reliability of epidemiological data.[3]
Chronic hoarseness is a prevalent voice complaint that significantly affects individuals' professional and social lives. In professions reliant on vocal function—such as teaching, call centers, or performing arts—hoarseness can hinder occupational functioning. Known causes of hoarseness include laryngeal inflammation, neoplasms, and endocrine conditions such as hypothyroidism and acromegaly [4]. Recent evidence has highlighted GERD and its subtype LPR as significant contributors to chronic hoarseness.
The aim of this review is to systematically examine the association between GERD and chronic hoarseness, with a focus on epidemiology, underlying pathophysiological mechanisms, diagnostic strategies, and treatment outcomes.
The novelty of this review lies in the integration of the most recent evidence up to 2025, its interdisciplinary perspective linking gastroenterology, otolaryngology, and speech-language pathology, and its focus on both pharmacologic and non-pharmacologic strategies, including voice therapy and emerging endoscopic techniques. This distinctive approach provides practical insights particularly relevant for professional voice users and other high-risk groups, offering a contribution not comprehensively addressed in earlier literature. These conclusions align with the stated aim of this review.
Hypothesis: The lack of standardized diagnostic criteria for laryngopharyngeal reflux significantly influences reported prevalence rates and treatment outcomes in patients with chronic hoarseness.
This narrative review was conducted in accordance with established guidelines for evidence-based literature synthesis. The literature search was performed in PubMed and Google Scholar. The initial protocol also included Scopus and Web of Science, but these databases were not used due to access limitations.
Search strategy and timeframe
The search covered articles published between January 2000 and April 30, 2025. The last search was conducted on April 30, 2025. The following keywords and Boolean operators were used:
A total of 4,532 records were identified. After removing 1,178 duplicates, 3,354 articles remained for screening by title and abstract. Of these, 60 full-text reports were assessed for eligibility, and 33 studies met the inclusion criteria and were included in the final analysis.
Peer-reviewed articles in English
Studies involving human subjects
Articles examining the link between GERD (or LPR) and hoarseness
Original research (RCTs, cohort studies, case-control studies) and systematic reviews
Non-English publications
Case reports, editorials, or letters without empirical data
Studies exclusively focused on pediatric populations or animal models unless directly related to GERD–hoarseness mechanisms
The quality of the included studies was assessed based on study design and adherence to established methodological standards. Randomized controlled trials and systematic reviews were considered the highest level of evidence, followed by prospective cohort and case-control studies. Observational studies were included when they provided relevant clinical or mechanistic data. Two reviewers independently evaluated study eligibility, and disagreements were resolved by discussion.
Relevant data were extracted manually and synthesized narratively. Data points included study design, sample size, GERD diagnosis methods, clinical presentation of hoarseness, interventions applied, and reported outcomes. Particular attention was given to the effectiveness of proton pump inhibitors (PPIs), anti-reflux surgery (ARS), and adjunctive therapies such as voice therapy and alginate-based treatments.
Based on the inclusion and exclusion criteria, a total of 33 studies were included in the final review. These encompassed randomized controlled trials (RCTs), observational studies (both prospective and retrospective), and systematic reviews.
The selected studies addressed a broad range of topics relevant to the association between GERD and chronic hoarseness, including:
Most studies originated from clinical gastroenterology, otolaryngology, and voice disorder literature, representing data across North America, Europe, and Asia. The included articles varied in sample size and methodology but contributed convergent findings suggesting a multifactorial association between GERD and chronic hoarseness. A qualitative synthesis of this evidence is presented in the subsequent section. Figure 1 illustrates the identification, screening, and inclusion process according to PRISMA guidelines
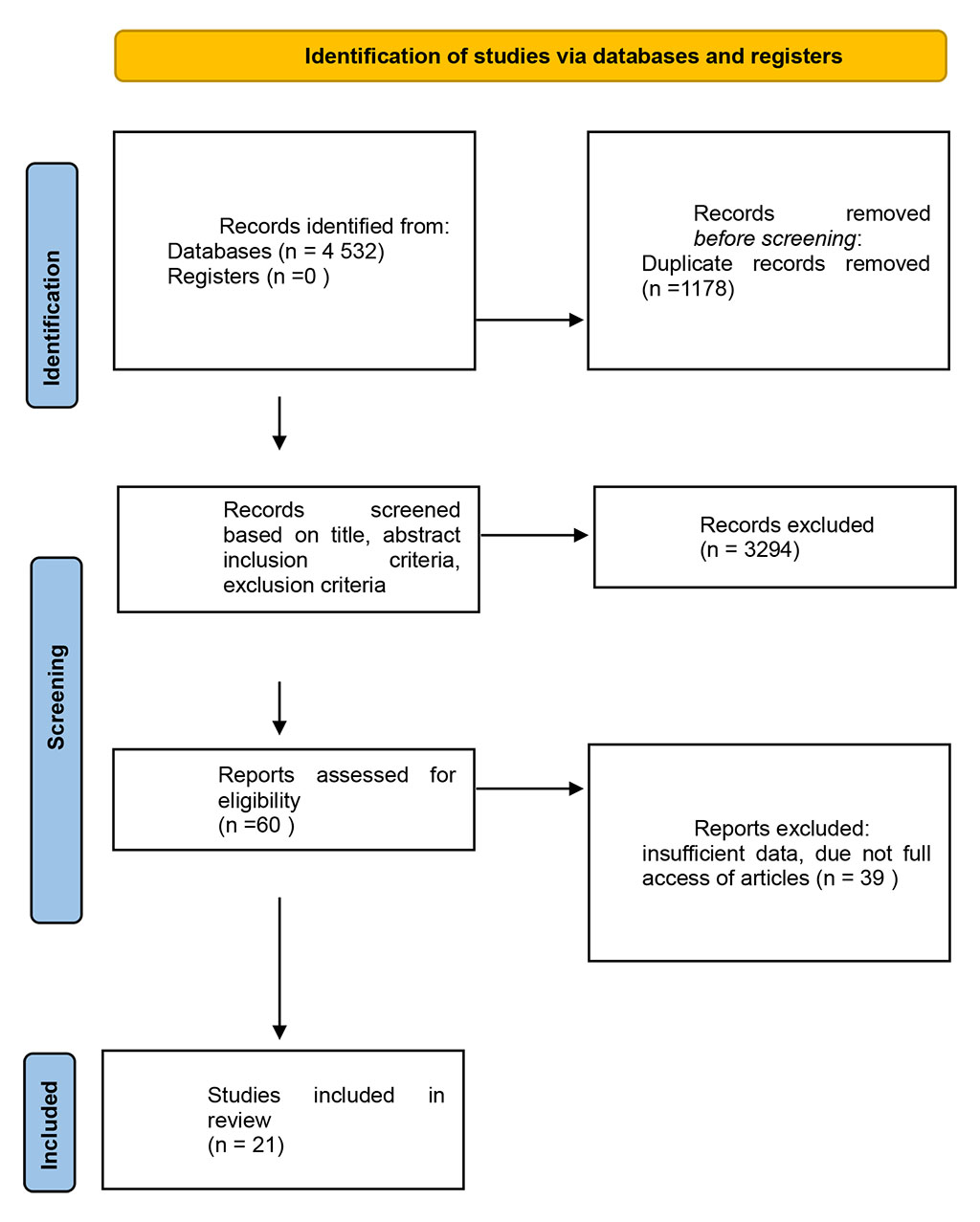
Figure 1. PRISMA flow diagram of study selection
The prevalence of hoarseness in the general population is approximately 6%, but this increases to 11% among professional voice users [4]. GERD is believed to contribute to a significant proportion of laryngeal complaints, with studies suggesting that reflux accounts for 10–50% of such cases [5]. In a prospective study involving 205 GERD patients, hoarseness was the most prevalent symptom, reported by 97% of participants [6]. Laryngopharyngeal reflux (LPR), a variant of GERD, has been directly associated with hoarseness, with symptom improvement noted after anti-reflux treatment [7], [5].
Demographic factors also influence hoarseness risk. Higher rates have been observed among older adults, children aged 8–14, and professional voice users such as teachers, telemarketers, and performers [4]. Women experience hoarseness 50% more often than men [4]. Furthermore, GERD may co-occur with serious conditions like laryngeal cancer, complicating diagnosis and treatment [8]. Supporting this, Baraka et al. (2025)[9] reported a high prevalence of GERD-related symptoms among Egyptian voice patients, with statistically significant correlations between reflux indicators and both perceptual and instrumental markers of dysphonia. Their findings underscore the clinical utility of integrated GERD screening in voice clinics to optimize diagnostic accuracy.
GERD arises from a dysfunction in the esophagogastric junction and lower esophageal sphincter (LES), leading to the backflow of stomach contents. Key contributing mechanisms include transient LES relaxations, reduced LES pressure, and delayed esophageal clearance [10], [11].
Postprandial “acid pockets” at the top of the stomach contents are significantly larger in GERD patients and are exacerbated by hiatal hernia, leading to prolonged acid exposure [12]. These factors contribute to reflux episodes even in upright positions.
Effective acid clearance requires intact esophageal motility and sufficient salivation. Impaired peristalsis and reduced salivary flow prolong acid contact with the mucosa, increasing damage risk [11].
Refluxed gastric contents (acid, pepsin, bile) penetrate the esophageal mucosa, disrupting tight junction proteins and widening intercellular spaces. This increases epithelial permeability and triggers local inflammation via cytokines such as IL-8 and IL-1β [13],[11]. Pepsin is especially damaging, as it can remain enzymatically active in weakly acidic environments and penetrate laryngeal tissue.
Inflammation sensitizes esophageal nociceptors, resulting in heightened perception of symptoms even without visible mucosal damage. This mechanism explains symptoms like globus and hoarseness in non-erosive reflux disease (NERD) [10].

Figure 2. Pathogenesis of GERD-related hoarseness
Figure 2. Proposed mechanisms linking gastroesophageal reflux disease (GERD) and chronic hoarseness. Acid and pepsin exposure cause direct mucosal injury and inflammation, while neurally mediated reflex pathways contribute to laryngeal hypersensitivity and altered vocal fold function. These parallel mechanisms explain the heterogeneity of clinical presentation in laryngopharyngeal reflux (LPR).
GERD-related hoarseness typically presents as a persistent alteration in voice quality, often described as raspy, breathy, or strained. Patients may also report throat clearing, chronic cough, globus sensation, or postnasal drip. These symptoms frequently occur without the hallmark features of GERD such as heartburn, complicating diagnosis.
A 2-week empirical trial of proton pump inhibitors (PPIs) is often recommended as a first-line diagnostic approach in patients with suspected GERD symptoms [1]. Improvement during the trial suggests acid-mediated etiology.
24-hour multichannel intraluminal impedance-pH (MII-pH) monitoring is the gold standard for detecting both acid and non-acid reflux, including gaseous episodes [13]. The 24-h MII allows for the assessment of refluxate based on composition (non-acidic pH > 7, weak-acidic pH 4-7, or acidic pH < 4) [16]. However, the high cost and patient discomfort limit widespread use. Although pH-impedance monitoring is regarded as the reference standard for evaluating reflux, complementary tools such as the Reflux Finding Score (RFS) and the Reflux Symptom Index (RSI) are commonly applied in clinical practice. However, several studies have demonstrated the limited specificity of RFS and the susceptibility of RSI to patient subjectivity, raising concerns about the reliability of these instruments in routine diagnostic use.[14], [15]
Upper endoscopy evaluates mucosal damage and identifies complications such as esophagitis or Barrett’s esophagus. Biopsy samples can exclude other etiologies and confirm inflammatory changes [12].
High-resolution manometry is employed to assess esophageal motility disorders and to guide the placement of pH probes [1]. It is essential before surgical interventions.
Laryngoscopic evaluation may reveal vocal cord edema, granulomas, contact ulcers, or subglottic swelling—signs consistent with LPR [13]. However, these findings are not specific, and differential diagnoses such as infection, allergy, and neoplasm must be ruled out.
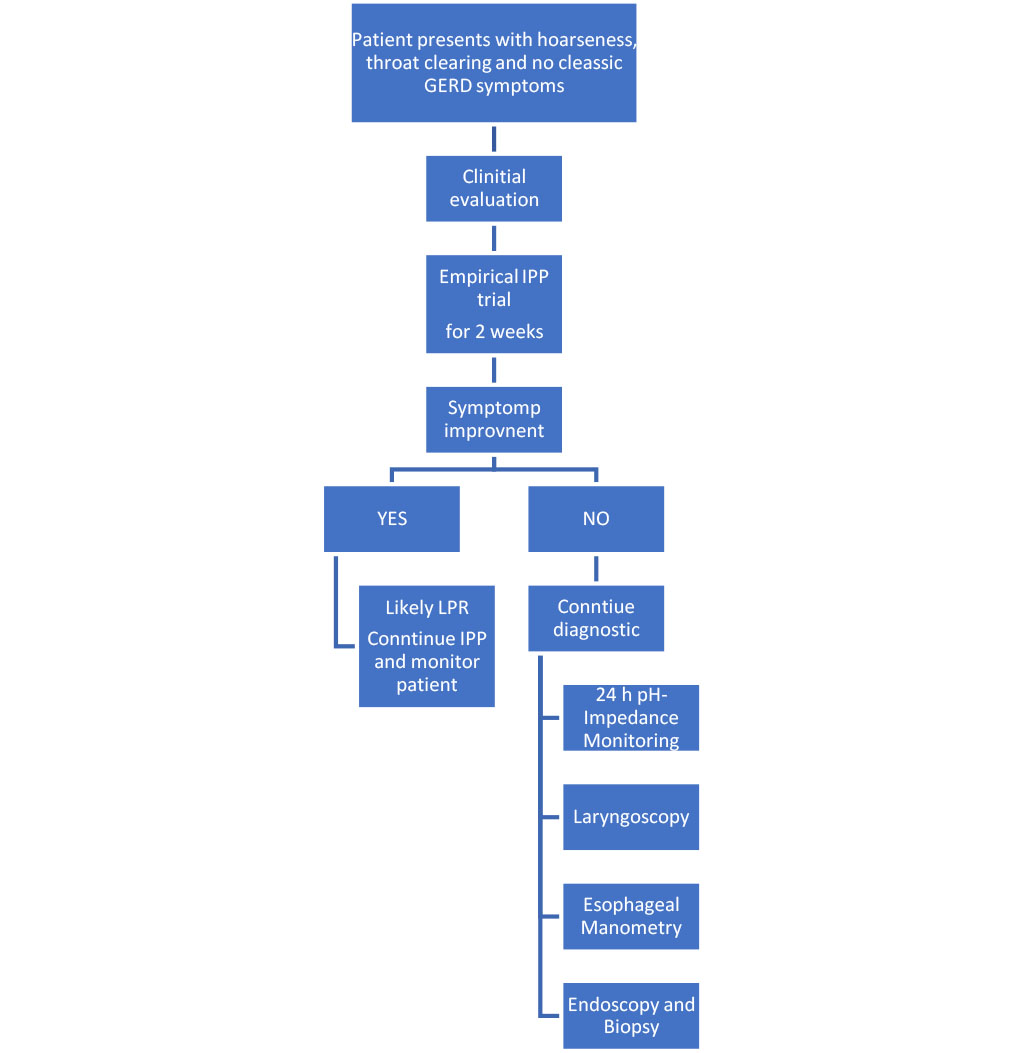
Figure 3. Diagnostic algorithm for GERD/LPR-related hoarseness
Figure.3 Suggested diagnostic approach for patients with chronic hoarseness suspected to be related to GERD or LPR. The initial assessment should include symptom-based screening (RSI, VHI) followed by a two-week PPI trial. In cases of persistent or unclear symptoms, laryngoscopy is indicated, and if uncertainty remains, pH-impedance monitoring and esophageal manometry are recommended. This stepwise approach supports targeted management and helps to avoid unnecessary interventions.
PPIs are the cornerstone of GERD treatment and are considered the first-line therapy for reflux-related hoarseness. They inhibit gastric acid secretion by irreversibly blocking the H+/K+ ATPase pump in gastric parietal cells.
Dosing and Response Rates
Common PPIs include omeprazole, esomeprazole, and pantoprazole. Patients are typically advised to take PPIs 30–60 minutes before breakfast. An initial 2–4-week trial is recommended, with dose adjustments based on symptom response [12].
Standard once-daily dosing achieves complete symptom relief in approximately 70–80% of patients with erosive reflux disease (ERD) and 60% in those with non-erosive reflux disease (NERD) [1]. However, 20–40% of patients do not adequately respond.
Efficacy for Hoarseness
A study by Pullarat et al. (2017) reported a 63% improvement in hoarseness among GERD patients after 8 weeks of PPI therapy. While some patients benefit from twice-daily dosing [17], [18], there is no evidence supporting more frequent dosing.
Limitations and Risks
Chronic PPI use has been associated with adverse effects including Clostridium difficile infection, pneumonia, chronic kidney disease, and osteoporosis-related fractures [12]. Therefore, long-term therapy should use the lowest effective dose. Although proton pump inhibitors (PPIs) remain the most commonly prescribed therapy for laryngopharyngeal reflux, their efficacy has shown considerable variability, with some studies reporting outcomes comparable to placebo. Moreover, while adjunctive approaches such as lifestyle modification, voice therapy, and endoscopic techniques can be beneficial, voice therapy in particular requires long-term adherence and may not provide rapid symptom relief. [19]
H2RAs inhibit gastric acid secretion by blocking histamine H2 receptors on parietal cells and are often used adjunctively with PPIs, particularly for nocturnal acid breakthrough (NAB).
Clinical Use
NAB refers to sustained periods of low gastric pH during the night despite PPI therapy. H2RAs such as ranitidine or famotidine are taken at bedtime to address this phenomenon [18].
Efficacy
While some randomized controlled trials (RCTs) suggest that adding H2RAs reduces nighttime acidity, others show limited benefit for GERD symptom control [1]. Tolerance can also develop with prolonged use.
PCABs are a newer class of acid-suppressing medications that act by reversibly inhibiting the gastric H⁺/K⁺ ATPase via competitive potassium binding. They differ from PPIs in their faster onset and independence from meal timing.
Examples and Use
Vonoprazan, tegoprazan, and fexuprazan are PCABs shown to be effective in both Asia and North America. These drugs are particularly beneficial for patients who are rapid PPI metabolizers or who have severe reflux esophagitis [18].
Efficacy
Meta-analyses have found that PCABs result in higher mucosal healing rates and symptom control than PPIs after 4–8 weeks, especially in Los Angeles grade C or D esophagitis [11]. They are also promising in PPI-refractory GERD.
Baclofen is a GABA-B receptor agonist that reduces the frequency of transient lower esophageal sphincter (LES) relaxations—the main mechanism behind reflux episodes.
Mechanism and Dosing
By modulating vagal control of the LES, baclofen decreases both acid and non-acid reflux episodes. Effective dosing ranges from 5–20 mg, taken three times daily [1].
Limitations
Though effective, its clinical use is limited due to central nervous system (CNS) side effects including sedation, dizziness, and short half-life [18]. It is not commonly prescribed outside of refractory cases.
Prokinetics enhance esophageal motility and gastric emptying, supporting acid clearance and LES function.
Adjunctive Role
Meta-analyses show that combining prokinetics with PPIs improves global GERD symptoms more than PPI monotherapy. Symptom relief increases from 50.6% with PPIs alone to 63.8% with the addition of prokinetics [1].
Recommendations
Prokinetics are especially useful in patients with suspected motility-related GERD or as part of a multi-targeted treatment plan [11].
Alginates are naturally occurring polysaccharides that form a gel-like raft on top of stomach contents, creating a physical barrier to prevent reflux [20].
Mechanism of Action
When taken postprandially, alginates float on gastric contents and neutralize pepsin and bile acids—major contributors to mucosal injury in GERD [10].
Efficacy
Meta-analyses demonstrate that alginate-containing compounds significantly outperform placebo and antacids in alleviating GERD symptoms, with a 60% symptom improvement rate [21]; [1]. Although slightly less effective than PPIs, they can be used as adjuncts, particularly at night [22]
Dosing
Typical regimens include 10–20 mL after meals and at bedtime.
Endoscopic interventions are minimally invasive options that aim to reinforce the LES or modify the reflux barrier.
TIF 2.0 creates a 3-cm valve with a 270° wrap, effective for regurgitation and patients with small (<2 cm) hiatal hernias. Studies show TIF provides better short-term symptom relief than PPIs [18].
This technique applies radiofrequency energy to the LES region to improve tone and reduce reflux. A meta-analysis of 28 studies showed improvement in heartburn, quality of life, and reduced PPI use [1].
ELGP involves internal plication of the stomach wall, shown to significantly reduce GERD symptoms and PPI dependence in PPI-resistant NERD [11].
Surgery is reserved for patients with severe GERD (e.g., Los Angeles grade C or D esophagitis) or those with PPI-refractory symptoms.
Laparoscopic Fundoplication
This is the gold standard surgical procedure, offering long-term symptom control. It involves wrapping the gastric fundus around the LES to augment reflux resistance. Studies report up to 90% symptom resolution 10 years post-surgery [12].
Effectiveness for Hoarseness
Meta-analyses show that anti-reflux surgery (ARS) results in significant improvement in respiratory symptoms, including hoarseness, with up to 80% of patients experiencing relief [23].
Risks
Complications include dysphagia (4.5%), abdominal distension, intraoperative bleeding, and emphysema. Careful patient selection is essential [13].
MSA is a newer, less invasive alternative using a magnetic ring to reinforce the LES. While promising, long-term data are limited, and MRI contraindications must be considered [12].
Lifestyle interventions are a foundational component of GERD management and can significantly reduce symptom frequency and severity—particularly in cases involving laryngopharyngeal reflux.
Recommended Strategies
Evidence of Effectiveness
In a retrospective study, patients receiving both pharmacologic and behavioral interventions showed significantly greater reductions in Reflux Symptom Index (RSI) scores compared to those receiving medication alone [13]. Although lifestyle changes alone may not be sufficient in severe GERD, they enhance treatment efficacy when used adjunctively.
Voice therapy is an evidence-supported adjunct to medical treatment, especially for patients whose hoarseness persists after acid suppression.
Mechanism and Role
Voice therapy targets vocal hygiene, breathing techniques, and phonation patterns. It can reverse maladaptive voice behaviors that may persist even after reflux is controlled.
Clinical Evidence
Implication
Since GERD-related dysphonia may not fully resolve with medical therapy, voice therapy should be incorporated into comprehensive treatment strategies, particularly in professional voice users or those with chronic symptoms.
Recent evidence by Al-Hussain (2024)[26] emphasizes the multidimensional benefits of voice therapy in treating dysphonia, particularly in patients with reflux-related vocal fold impairments. The review highlights that targeted voice therapy techniques—including semi-occluded vocal tract exercises and resonant voice therapy—can yield substantial improvements in phonatory stability and perceptual voice quality even in cases where pharmacological intervention alone is insufficient.
Table 1 summarizes the main pharmacological, surgical, and behavioural treatment options with their efficacy and clinical notes
Table 1. Key treatment modalities in GERD-related hoarseness
| Treatment Modality | Mechanism | Efficacy | Dosing/Procedure | Clinical Notes |
| Proton Pump Inhibitors | Gastric acid suppression | 70-80% ERD, 60% NERD | 30-60 min before first meal, 2-4 weeks initial | First-line therapy, 20-40% non-responders |
| Histamine-2 Antagonists | Histamine receptor blockade | Variable for nocturnal breakthrough | Bedtime dosing with PPI | Adjunctive for night symptoms |
| Potassium-Competitive Acid Blockers | Reversible potassium pump inhibition | Comparable to PPIs | Vonoprazan 20mg/day, 4-week course | Effective in PPI metabolizers |
| Baclofen | GABA-B agonist, reduces LES relaxations | Effective for refractory cases | 5-20mg three times daily | CNS side effects limit use |
| Prokinetics | Enhanced esophageal motility | 63.8% combined vs 50.6% PPI alone | Combined with PPI therapy | Significant improvement in global symptoms |
| Alginate-based Therapy | Creates protective gastric "raft" | 60% improvement vs placebo | 10-20mL after meals, bedtime | Neutralizes pepsin and bile acids |
| Laparoscopic Fundoplication | Anti-reflux valve creation | 90% relief at 10 years, 80% respiratory improvement | Surgical procedure | Superior to medical therapy, 4.53% dysphagia |
| Magnetic Sphincter Augmentation | LES function augmentation | Comparable to fundoplication | Minimally invasive placement | MRI contraindicated post-procedure |
| Transoral Incisionless Fundoplication | 270° esophageal wrap | Superior to PPIs short-term | Endoscopic 3cm valve creation | Effective for regurgitation, <2cm hernias |
| Stretta Procedure | Radiofrequency energy delivery | Improved HRQOL, reduced PPI use | Endoscopic radiofrequency application | 28 studies, 2468 patients analyzed |
| Voice Therapy | Vocal rehabilitation | Combined therapy superior to monotherapy | 3-6 month structured program | Addresses persistent dysphonia |
| Lifestyle Modifications | Multiple mechanisms | Supportive, variable efficacy | Weight loss, dietary changes, positioning | Cost-effective, no adverse effects |
Medical Treatment Success Rates
The effectiveness of GERD treatment in managing hoarseness varies across populations and treatment modalities. PPIs remain the most widely used therapy, though response rates are inconsistent.
Pharmacologic Therapy
Surgical Outcomes
Quality of Life Impact
Hoarseness associated with GERD significantly affects patients’ daily functioning, particularly in professional and social settings.
Voice-Related Quality of Life (VR-QoL)
Effect of Treatment
The reviewed literature demonstrates a consistent association between gastroesophageal reflux disease (GERD) and chronic hoarseness. Multiple studies indicate that GERD, particularly its variant laryngopharyngeal reflux (LPR), can cause voice disturbances through direct mucosal injury, chronic inflammation, and neural sensitization [28]. Reflux-related hoarseness is frequently underdiagnosed, especially in the absence of typical symptoms such as heartburn, underscoring the importance of increased clinical awareness.
Diagnostic Challenges and Approaches
Diagnosis of GERD-related hoarseness relies on a combination of approaches, including empirical proton pump inhibitor (PPI) trials, pH-impedance monitoring, endoscopy, laryngoscopy, and manometry. However, the absence of a universally accepted gold standard for extraesophageal manifestations remains a significant diagnostic challenge, highlighting the need for standardized protocols. This diagnostic variability is further complicated by the considerable differences in reported prevalence rates of LPR between countries, largely due to variations in diagnostic criteria and assessment methods [29]. These differences significantly influence epidemiological data and limit comparability between studies.
Therapeutic Strategies and Outcomes
Current therapeutic strategies most often include PPIs as first-line treatment, although their efficacy in LPR has shown considerable variability, with some studies reporting outcomes similar to placebo. This has led to increased interest in adjunctive and alternative therapies, such as potassium-competitive acid blockers (PCABs), baclofen, prokinetics, and alginate-based formulations [30]. Voice therapy, while effective in selected cases, requires long-term adherence and may not result in rapid symptom improvement. For patients with refractory disease or anatomical factors such as large hiatal hernias, anti-reflux surgery (ARS) has shown favorable results.
Multidisciplinary Management Considerations
Management is most effective when multidisciplinary, involving gastroenterologists, otolaryngologists, and speech-language pathologists [30]. However, access to such integrated care may be limited in some healthcare systems due to organizational and economic constraints, which can affect the practical application of these recommendations. The combination of pharmacologic treatment, lifestyle modifications, and behavioral interventions increases the likelihood of symptom resolution and improved quality of life.
Although a multidisciplinary approach involving gastroenterologists, otolaryngologists, and speech-language pathologists is widely regarded as the optimal management strategy, its practical implementation is frequently constrained by organizational and economic factors, limiting feasibility in many healthcare systems.[31]
Study Limitations
This review has several limitations that should be acknowledged. The absence of a predefined cut-off date for the literature search may have led to the omission of the most recent publications at the time of writing. The restriction to articles available in English could have excluded relevant studies published in other languages. Additionally, the selection and interpretation of data may be subject to selection bias, and the heterogeneity of study designs and diagnostic criteria across included sources limits the comparability of findings.
GERD-related hoarseness represents a clinically significant condition that warrants early detection and targeted management. The findings of this review support the hypothesis that the absence of standardized diagnostic criteria for LPR significantly contributes to variability in reported prevalence rates and treatment outcomes in patients with chronic hoarseness. Standardization of diagnostic protocols is essential to improve the accuracy of epidemiological assessments and to guide therapeutic strategies.
Optimal management requires a personalized approach that may combine pharmacological therapy, surgical intervention when indicated, and behavioral modifications, supported by multidisciplinary collaboration. These conclusions are consistent with the stated aims of this review and are supported by recent studies including Zheng et al. (2024)[28], Lechien et al. (2021)[30], Kamani et al. (2012)[29], Huang et al. (2022)[32], and Lechien (2025)[33].
These findings confirm the stated hypothesis and reinforce the need for standardized diagnostic protocols to improve both epidemiological reliability and therapeutic outcomes
Artificial intelligence tools (ChatGPT, OpenAI) were used to support language editing, formatting suggestions, and refinement of structure according to reviewers’ comments. The content, analysis, and conclusions remain the responsibility of the authors, who verified all information against the cited sources