- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 4. DOI 10.35630/2024/14/4.408
Oral cancers are seen frequently. By 2040, according to the Global Cancer Observatory (GCO), their number will increase by around 40%. Exposure to alcohol, tobacco smoke, human papillomavirus (HPV) or a diet low in fibre is often part of everyday life, which is a risk factor for developing oral cancer. The most common of these is oral squamous cell carcinoma. The most common treatment is primary surgical resection with or without postoperative adjuvant therapy. In addition, various combination therapies including chemotherapy with radiotherapy or immunotherapy are used. Oral cancers are usually detected early but delayed treatment can lead to a bad prognosis and require facial reconstructive surgery.
Aim: The aim of the publication was to bring together the latest information on oral cancer in Poland. We wanted to present the division, the most commonly observed symptoms and the proposed treatment.
Methods: Analysis of information on oral cancer using PubMed sources, including classification of cancer types, symptoms and possible treatments.
Conclusions: Oral cancers are a common problem for the population of Poland. Their incidence is likely to increase over the next decade. The environment, lifestyle and diet have a key influence on their formation. The most common treatment is their resection combined with radiation therapy, chemotherapy or immunotherapy.
Keywords: oral cavity cancer; oral cancer; squamous cell carcinoma; head and neck cancer
According to the International Agency for Research on Cancer WHO Global Cancer Observatory, the incidence of lip and oral cavity cancers (ICD code C00-C06) in Poland was 4081 cases, while the incidence of salivary glands cancers (ICD code C07-C08) was 530. Thus, 2.28% represent all cancers excluding non-melanoma skin cancer. In 2021, mortality statistics for oral and pharyngeal cancers in Poland were 2,300. Laryngeal cancer is responsible for most deaths followed by tongue cancer and finally lip cancer. Approximately 69% of cases occurred in men. [7] The number of oral cancers diagnosed has increased over the past 20 years, according to the Polish National Cancer Registry. Over the past 10 years, the incidence of oral and neck cancers in Poland has increased by about 25%. [8]
Poland stands out among European Union countries due to its high burden of oral cancers. In addition, the incidence rate of head and neck cancers in Poland is about 1.4 times higher for men and 1.2 times higher for women compared to the average in other European Union member-states. [9] The main risk factors for oral cancers can be smoking and alcohol consumption [10], so this significant burden may be attributed to the higher prevalence of tobacco and alcohol consumption in Poland compared to Western European countries. [11,12] As many as 80% of head and neck cancers can be linked to smoking(13) and it is calculated that 21% of Poles aged 15 years and older smoke every day and almost 600,000-700,000 are alcohol addicted, what seems to be real problem. [9,14,15]
For comparison, in Europe lip, oral cavity and salivary glands cancers is being accounted for 1.71% of all cancers diagnosed. This statistic indicates how big the problem of oral cavity cancers stands in Poland comparing to the rest of Europe. Worldwide these cancers accounted for 2.37%. Worldwide men were twice as likely as women to develop these cancers. Due to the lack of detailed data on the incidence of each type in Poland, we often had to limit ourselves to worldwide data.
The aim of our work was to bring together the latest information on oral cancer in Poland. We wanted to present the division, the most commonly observed symptoms and the proposed treatment.
Oral cancer is a malignant neoplasm that localizes on the lip or in the oral cavity. Oral cancers are a very heterogeneous group of cancers that have a varying propensity to metastasize to lymph.[1,2] More than 90% of oral cancers are squamous cell carcinoma (SCC). It is the most studied malignant tumor of the head and neck region worldwide.[3]Other non-squamous oral cancers are rare and include malignant tumors of small salivary glands, lymphomas, intraoral sarcomas, malignant odontogenic tumors, melanomas or metastases from distant tumors.[4–6]
Oral squamous cell carcinoma is a malignant neoplasm that originates from dysplasia of the epithelium lining the oral cavity. It is the most common oral cancer and accounts for about 90% of cases in Poland.[1,16,17] OSCC locally destroys the subepithelial basement membrane through tumor proliferation.[16] Invasion of neoplastic epithelial cells through the basement membrane into the superficial connective tissue is a criterion for the diagnosis of oral squamous cell carcinoma.[18] The WHO International Classification of Tumors[2] divides oral cancers based on the degree of differentiation into well-differentiated, moderately differentiated, poorly differentiated or undifferentiated. The degree of differentiation of a tumor determines its ability to form metastases.[16] In addition to conventional oral squamous cell carcinoma, there are:[5,18]
Malignant tumors of the small salivary glands are the second most common malignant tumors in the oral cavity.[19] Tumors of salivary glands account for less than 5% of oral cancers. The most common location in the oral cavity is the hard palate (60%), lip (25%) and buccal mucosa (15%).[6] The two most common types of cancer are pleomorphic adenoma and mucoepidermoid carcinoma. [20] It is most common in the 5th to 7th decade of life, more common in women.[20]
Oral lymphomas are the third most common malignancy of the oral cavity, second only to squamous cell carcinoma and malignant tumors of the small salivary glands. Oral lymphomas are rare and account for only 3% of all lymphomas in the general population. [19] Lymphomas are malignant tumors of lymphocytes and their precursors. [21] They occur in two forms: Hodgkin's lymphoma (HL) and non-Hodgkin's lymphoma (NHL). Non-Hodgkin's lymphoma occurs in extra-nodal locations in 40% of cases, while Hodgkin's lymphoma occurs in only 1-4% of cases. The head and neck region is the second most common location for non-Hodgkin's lymphoma. In the head and neck region, the most common location of lymphomas is the tonsils, followed by the salivary glands. [19] In the oral cavity, it is the palate, gums, tongue, buccal mucosa, floor of the mouth and lips, as primary sites in about 2% of cases of extranodal lymphomas. [21] The most common histologic type is diffuse large B-cell non-Hodgkin’s lymphoma (DLBCL). Other types of lymphoma include small cell non-Hodgkin's lymphoma and Burkitt's lymphoma. [19]
Sarcomas are malignant tumors that derived from cells of mesenchymal origin. The head and neck regions account for 4-10% of cases of sarcomas throughout the body. Oral sarcomas are even rarer. [22] They originate in fibrous, muscular, bony, cartilaginous, adipose, vascular and nervous tissue.[22,23]
The most common type is rhabdomyosarcoma (RMS), which accounts for 18% of soft tissue sarcomas of the head and neck. The next types of sarcomas are malignant fibrous histiocytoma, fibrosarcoma and neurofibrosarcoma. Other sarcomas account for 13% of head and neck sarcomas.[23] Kaposi's sarcoma is a mucocutaneous neoplasm. It localizes in the oral cavity most often in cases of AIDS-KS. The presence of such a sarcoma may signal undiagnosed HIV infection. The most common localization of Kaposi's sarcoma is the hard palate, soft palate, gingiva and dorsum of the tongue.[24]
Odontogenic tumors are a group of lesions including malformations of tooth tissues, benign and malignant tumors. Odontogenic tumors originate in the tissues from which teeth are formed, so they are mainly jaw lesions, but some appear as localized gingival swellings (peripheral odontogenic tumors).[25] Most odontogenic tumors are benign, among which ameloblastoma is the most common. It accounts for about 1% of all cysts and tumors occurring in the jaws. It arises from dental embryonic remnants. Ameloblastoma is divided into two types: the conventional type - solid/multicystic variant and the unicystic type.(26) The solid/multicystic variant is considered a locally invasive tumor and has the ability to locally infiltrate into the neighboring bone marrow. It should be considered potentially malignant when planning surgical treatment [25]. Peter R. Morgan [25] divides malignant odontogenic tumors into:
Primary oral melanoma (POM) is a rare cancer with an aggressive course and poor prognosis. The secondary form (metastasis) is even rarer. Oral melanoma arises from neoplastic transformation of mucosal melanocytes. It accounts for 0.5% of all oral cancers and 0.2-8% of melanomas of the whole body. In 80% of cases, oral melanoma occurs in the maxilla and in 20% in the mandible.[27,28] The most common location is the hard palate and gingiva. Other sites include the tongue, mandible, buccal mucosa and upper and lower lip. Primary melanoma is more common on the hard palate and mandible, while secondary melanoma occurs on the tongue, parotid gland and tonsils.[28]
The early stages of squamous cell carcinoma of the oral cavity are typically asymptomatic due to the small size of the neoplastic lesions.[6] Malignancy should be suspected if an oral lesion persists for more than three weeks.[1,6,29,30] Therefore, it is crucial to perform a thorough examination of the oral mucosa, with particular attention to the tongue, floor of the mouth, and lower lip. Palpation of the cervical and head lymph nodes is recommended to detect possible metastases. [1,6,29,30]
The most common symptom of squamous cell carcinoma is pain, which typically arises when the neoplastic lesions become larger, prompting patients to seek medical consultation.[29] This pain predominantly affects the tongue and the floor of the mouth, leading to impairment in speech, chewing, and swallowing.[31–33] In more advanced stages of the disease, irregularly bordered ulcers, which are hard and painful upon palpation, may appear, along with bleeding and issues with dental prosthesis fitting.[6,34,35] Less common symptoms include paresthesia, numbness of the chin area, voice changes, ear inflammation, and significant weight loss.[30,34]
Lymphomas of oral cavity are characterized by significant swelling and the presence of a tumor, which may present with or without ulceration. The onset of the disease is often asymptomatic, complicating accurate diagnosis, as early symptoms can resemble other benign conditions.[4,19,36] In the later stages of the disease, extensive areas of necrosis and purulent inflammation with vascular infiltration are observed.[21] Additionally, symptoms may include paresthesia, anesthesia, tooth loss, ulcerations, pain, facial asymmetry, and enlargement of the cervical and submandibular lymph nodes.[4,19]
The symptoms of oral sarcomas are varied, primarily involving tender swelling. Additionally, pain, ulcerations, tooth mobility, and paresthesia may occur.[24,37] Lesions can manifest as single or multifocal. When ulceration or secondary infection with oral flora occurs, additional symptoms such as bleeding and suppuration may appear.[24]
Carcinoma of the minor salivary glands of the oral cavity may present as a painless swelling or mass, causing symptoms related to the compression of adjacent structures, such as pain or nerve paralysis.[38] The mass may be present with or without ulceration, potentially leading to symptoms such as dysphagia and dysphonia.[39]
The symptoms of odontogenic tumors are nonspecific.[40] Intrabony lesions associated with these tumors can lead to pain and sensory disturbances in the area innervated by the trigeminal nerve.[4] Other common yet nonspecific symptoms of odontogenic tumors include swelling, tooth mobility, and pathological fractures.[40]
Clinically, oral melanomas are characterized by an asymmetrical shape, single or mixed coloration of black, brown, gray, purple, red. Some are amelanotic and this can cause difficulties in diagnosis.[27,28] Routine biopsy of questionable pigmented lesions is recommended.[27]
Two most significant risk factors for the development of oral cancer (OC) are use of tobacco products and alcohol consumption. Additionally, factors such as betel quid chewing, UV radiation exposure, viral infections, oral hygiene, and dietary factors also play a role.
Major risk factors involved in the development of OC are listed in the Figure 1.
Smoking is the strongest risk factor for the development of oral cancer. Tobacco smoke contains over 9,000 substances, including aromatic hydrocarbons, amines, N-nitrosamines, phenols, cadmium and arsenic compounds, 83 of which are proven carcinogens.[41] Some of these substances have oxidative potential on their own or produce intermediate compounds during their metabolism, exerting carcinogenic effects through oxidative DNA modifications in exposed cells.[42] Smoking is associated with more than a threefold increase in the risk of developing oral cancer compared to non-smokers, with a particular increase in the incidence of squamous cell carcinoma (SCC).[43]Passive smoking also shows a correlation with the development of oral cancer, although this link is weaker.[44] After smoking cessation, the risk of developing oral cancer decreases - by 30% after 4 years compared to continuous smokers, and reaching a risk similar to the non-smoking population after 20 years of cessation.[45] Additionally, smoking promotes the development of periodontal disease, which is also associated with the development of oral cancer.[44] Other forms of tobacco use also contribute to carcinogenesis - the popular practice of chewing tobacco products in South American and Southeast Asian countries is associated with an increased relative risk of developing oral cancer with RR of 3.64 (95% CI 3.00-4.41).[46]
Alcohol consumption is an independent risk factor for the development of oral and pharyngeal cancer, with the risk increasing with daily alcohol intake.[47,48] The basis of alcohol's carcinogenic action lies in its direct effect on oral epithelial cells and chemical damage to the oral mucosa, facilitating the penetration of other carcinogens. Besides, alcohol, acting as a solvent for other carcinogenic substances, allows for their easier penetration into the mucosa compared to non-dissolved forms. The relative risk of developing oral cancers is estimated at 1.75 (95% CI 1.70-1.82) for 25 g of pure alcohol per day, 2.85 (95% CI 2.70-3.04) for 50 g per day, and 6.01 (95% CI 5.46-6.62) for 100 g per day.[49] There is also a link with the development of head and neck cancer (HNC) for mouthwash products containing alcohol, but only in combination with other risk factors for this disease.[50] Smoking tobacco products and alcohol consumption have a cumulative effect and together lead to a multiple - estimated up to 35-fold—increase in the risk of developing oral cancer.[51] These two factors alone are responsible for up to 70% of head and neck cancer cases.[52]
Human papillomavirus (HPV) infection is another risk factor for the development of oral cancer. HPV belongs to the Papillomaviridae family, is a DNA virus, and is transmitted through sexual contact or vertically. The risk of HPV infection increases with the number of sexual partners, with the highest risk of transmission being unprotected oral-genital intercourse.[53] Patients suspected of having an HPV-related cancer are younger than the general population of OC patients and have lower exposure to tobacco smoke and ethanol. HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 are considered high-risk for oncogenic transformation, with HPV 16 being the most frequently detected subtype in SCC of the head and neck.[54] The presence of any high-risk HPV genotype is positively correlated with the development of head and neck cancer (HNC) - OR 1.83 (95% CI 1.04-2.62) compared to HPV-negative individuals.[55] Up to 23.5% of oral SCCs are associated with HPV infection.[56] The population of patients with HPV as the main cause of SCC of the head and neck has better treatment outcomes compared to the general population with this diagnosis.
Betel nut (betel quid) is a stimulant and narcotic used commonly in Southeast Asia, consisting of betel leaves and areca nut seeds, sometimes with added tobacco. The main carcinogen in areca nut seeds is arecoline, which has proven DNA-damaging effects on human epithelial cells.[50] Betel chewing is associated with a relative risk of developing oral cancer—2.56 (95% CI 2.00-3.28). This risk increases with the amount of product consumed. If tobacco is added to the consumed substance, the relative risk can be as high as 7.74 (95% CI 5.38-11.13).[58]
Poor oral hygiene and related periodontal diseases, such as gingivitis and periodontitis, are associated with both local and systemic inflammation and are correlated with the development of cancers of all kinds.[59] Periodontal diseases, including advanced periodontal disease and its indirect indicator, tooth loss, are positively correlated with the development of head and neck cancers.[60] Tooth loss is associated with a 2-3-fold increase in the risk of developing oral cancer, even considering other risk factors such as smoking and alcohol consumption, although these risk factors, when coexisting, can synergistically promote the development of oral cancers.[61]
UV radiation is a well-documented risk factor for the development of skin cancer, particularly melanoma. In the context of oral cancer, UVB radiation plays a role in the development of lip cancer. The incidence of lower lip cancers is significantly higher than upper lip cancers, most likely due to the greater amount of radiation received by the lower lip.[62]
Epstein-Barr virus (EBV) is a DNA virus from the Herpesviridae family, commonly found in the global population.[63] In addition to acute infection presenting as infectious mononucleosis, it causes latent infections with the main reservoir being infected B lymphocytes and, to a lesser extent, oral epithelial cells.[64] EBV is implicated to play a role in the development of several neoplasms, including Burkitt's lymphoma, nasopharyngeal carcinoma, and oral squamous cell carcinoma.[58] EBV infection is associated with an increased risk of developing oral SCC - OR 2.57 (95% CI 1.23-5.36).[66]
Dietary factors are also associated with head and neck carcinoma. Higher consumption of red meat and processed meat is positively correlated with the development of HNC - OR 1.40 (95% CI 1.13-1.74) and 1.37 (95% CI 1.14-1.65), respectively. Conversely, higher consumption of vegetables and fruits is inversely correlated with HNC development - OR 0.52 (95% CI 0.43-0.62).[67]
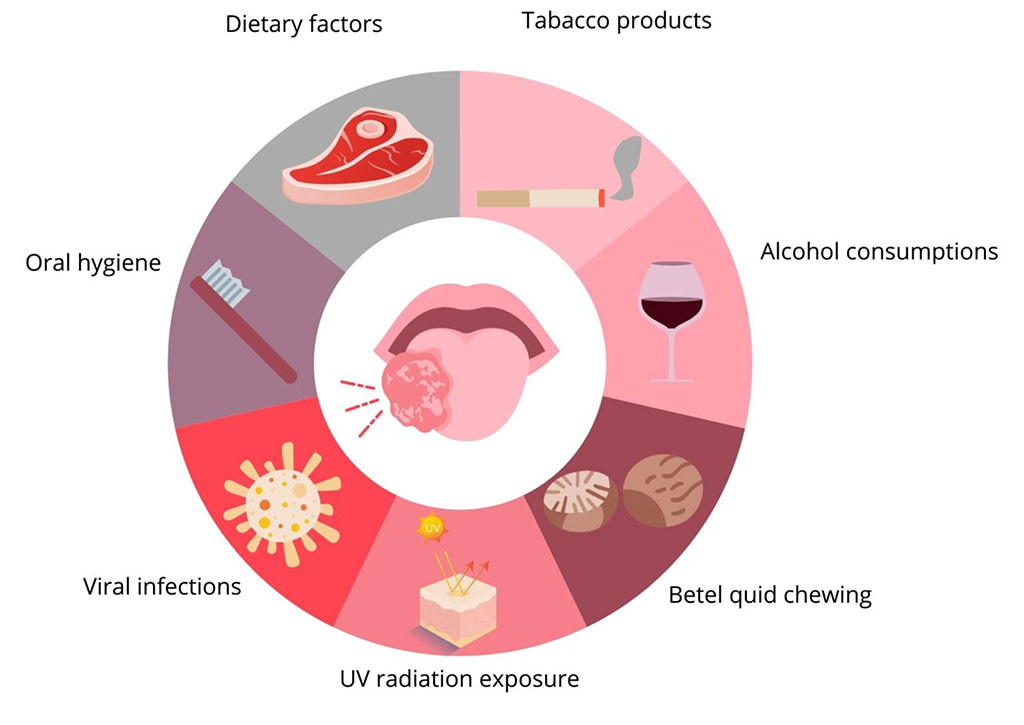
Figure 1. Risk factors for development of oral cancer
In the case of squamous cell carcinoma, the management consists of surgical removal of the lesion, with the necessity of removing a margin of healthy tissue. When, due to anatomical conditions, it is not possible to obtain an adequate margin or metastases are located in the removed lymph nodes, postoperative radiotherapy is additionally used.[6] Chemotherapy can be used early in the treatment or as an adjunct therapy to surgery and/or radiotherapy.[6,68]
The management of oral sarcomas consists of surgical removal of the lesion along with a wide margin; in addition, treatment may be supplemented with radiation therapy. For striated sarcoma, treatment includes both surgical excision of the lesion and complementary chemotherapy.[69–71]
Both Hodgkin's lymphoma and oral non-Hodgkin's lymphoma respond well to treatment with chemotherapy, especially if the lesion is located within the bone, in which case chemotherapy may be used as the sole treatment modality.[72] If diagnosed early in the course of non-Hodgkin's lymphoma, radiation therapy may be a stand-alone treatment option. For aggressive lymphomas, chemotherapy and radiation therapy are used simultaneously.[72–74]
In most cases, treatment consists of surgical removal of the tumor, along with partial or complete resection of the maxilla or mandible, depending on the location of the lesion.[75] In the case of metastasis to the surrounding lymph nodes, removal is also necessary.[76]
The primary treatment for oral mucosal melanoma is surgical excision of the lesion along with a margin of the surrounding soft tissues ranging from 1.5 - 2 cm.[77] If adequate margins of the surrounding soft tissues cannot be obtained, or if the tumor is significantly advanced, adjuvant treatment also includes chemotherapy, radiation therapy and immunotherapy.[78]
Oral cancer is a serious problem for our society. In recent years, there has been a growing trend in the incidence of oral cancer in Poland. The likely reason is exposure to risk factors, which, along with changes in the lifestyle of Poles, favor their incidence and exposure. The most common cancer is oral squamous cell carcinoma, the occurrence of which is promoted by smoking, alcohol consumption and viral infections. Cancer prevention is an important factor in reducing the risk of the disease. In addition, oral cancer data shows late detection of cancer and the population is not sufficiently aware of the disease.