- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 4. DOI 10.35630/2024/14/4.402
One of the main causes of blindness in the elderly in developed countries is age-related macular degeneration (AMD) [1, 2, 3]. Active research is being conducted to identify putative molecular targets for the development of an effective strategy for conservative treatment of both atrophic and neovascular forms of this disease [4, 5, 6], but at the present stage, pharmacotherapy is effective only for some patients, and is insufficient to stop the progression of the disease or eliminate the damage that has already occurred. The most promising and promising potential treatment options are considered to be the introduction of cellular technologies with retinal cell replacement and targeted pharmacotherapy.
The aim of this study was to investigate the role of the retinal development inducer, SIRT1 [7, 8, 9], in retinogenesis to justify pharmacological inhibition of this protein as a possible treatment option for neovascular AMD.
Using immunohistochemistry and immunocytochemistry, we assessed the expression and subcellular localization of SIRT1 and its innate inhibitor DBC1 in the retina of fetuses, adults, and mice. We also studied SIRT1 in retinal progenitor cells derived from mice and humans, the former having been used in small interfering RNA studies. SIRT1 is widely expressed in the developing and adult retina and is a regulator of key genes in retinal development, namely PAX6, Nestin, and CRX [10, 11, 12]. Moreover, we found that photoreceptor progenitor cells were among the smallest cells in a heterogeneous mouse retinal progenitor cell population [13, 14, 15]. Collectively, these results provide a basis for manipulating SIRT1 expression in small retinal progenitors as a means to increase the yield of photoreceptors for transplantation in retinal degeneration models [16, 17]. Furthermore, SIRT1 was found to be highly expressed in human-derived choroidal neovascular membranes, allowing its activity to be pharmacologically inhibited by the drug nicotinamide as a potent regulator of angiogenic and hypoxic signaling in a human retinal pigment epithelial cell line at both the protein level using angiogenesis arrays and at the RNA level using whole genome microarrays [18, 19]. These results point to the SIRT1 inhibitor, Nicotinamide, as a possible agent for treatment of neovascular AMD. Further studies of Nicotinamide are warranted in animal models of AMD. To the best of our knowledge, this is the first time that a detailed analysis of SIRT1 as a regulator of both retinal development and choroidal neovascularization has been reported.
Keywords: AMD, immunocytochemistry, SIRT1, Nicotinamide, neovascularization
The role of SIRT1 (Sirtuin 1) in retinal development and its potential as a therapeutic target in age-related macular degeneration (AMD) have garnered significant attention in scientific research. SIRT1 is a member of the sirtuin family of proteins, which are involved in various cellular processes such as gene regulation, DNA repair, metabolism, and aging.
Regulation of Retinal Development: SIRT1 has been implicated in the regulation of retinal development, particularly in processes such as cell differentiation, survival, and synaptic function. Studies have shown that SIRT1 is expressed in various cell types within the retina, including retinal ganglion cells, photoreceptors, and Müller glial cells. It plays a role in modulating gene expression patterns that are crucial for the development and maintenance of retinal structure and function.
Therapeutic Target in AMD: Age-related macular degeneration (AMD) is a leading cause of vision loss among the elderly, characterized by degeneration of the macula, the central part of the retina. Oxidative stress, inflammation, and dysfunction of the retinal pigment epithelium (RPE) are implicated in the pathogenesis of AMD. SIRT1 has emerged as a potential therapeutic target for AMD due to its involvement in pathways related to oxidative stress, inflammation, and cellular senescence.
Oxidative Stress: SIRT1 has antioxidant properties and can modulate cellular responses to oxidative stress by activating antioxidant defense mechanisms and promoting mitochondrial function.
Inflammation: SIRT1 exerts anti-inflammatory effects by inhibiting the NF-κB pathway and modulating the activity of inflammatory mediators. In AMD, chronic inflammation contributes to disease progression, and targeting SIRT1 may help mitigate inflammatory responses in the retina.
Cellular Senescence: SIRT1 plays a role in regulating cellular senescence, a state of irreversible growth arrest associated with aging and age-related diseases. Dysregulated senescence in RPE cells is implicated in AMD pathogenesis, and SIRT1 activation may alleviate senescent phenotypes and promote RPE cell survival.
Therapeutic Strategies: Several therapeutic strategies targeting SIRT1 have been explored for the treatment of AMD, including pharmacological activation of SIRT1 using small molecule activators or dietary interventions. These approaches aim to enhance SIRT1 activity in the retina to counteract oxidative stress, inflammation, and cellular senescence, thereby preserving retinal structure and function in AMD.
Overall, the dual role of SIRT1 as a regulator of retinal development and a potential therapeutic target in AMD underscores its importance in maintaining retinal homeostasis and suggests its therapeutic potential for mitigating age-related retinal degenerative diseases. Further research is needed to elucidate the precise mechanisms underlying SIRT1's actions in the retina and to evaluate the efficacy and safety of targeting SIRT1 in clinical settings.
The study on the dual role of SIRT1 in regulating retinal development and its potential as a therapeutic target in age-related macular degeneration (AMD) is highly relevant for several reasons:
Understanding Retinal Development: Investigating the role of SIRT1 in retinal development provides insights into the molecular mechanisms underlying the formation and maintenance of the retina. This knowledge is crucial for understanding normal retinal physiology and pathophysiology, which is essential for developing effective treatments for retinal disorders.
Identifying Therapeutic Targets for AMD: AMD is a prevalent cause of vision loss, particularly in the elderly population. Current treatment options for AMD are limited, and there is a growing need for novel therapeutic targets. Studies on SIRT1 highlight its potential as a therapeutic target for AMD by modulating pathways related to oxidative stress, inflammation, and cellular senescence, which are implicated in AMD pathogenesis.
Developing Therapeutic Strategies: Research on SIRT1 opens avenues for developing therapeutic strategies aimed at enhancing SIRT1 activity in the retina. Pharmacological agents or dietary interventions that activate SIRT1 could potentially slow down the progression of AMD and preserve retinal function. Understanding the mechanisms through which SIRT1 exerts its effects in the retina can inform the development of targeted therapies for AMD.
Implications for Age-Related Diseases: AMD shares common pathogenic mechanisms with other age-related diseases, such as neurodegenerative disorders and cardiovascular diseases. Therefore, insights gained from studying SIRT1 in the context of AMD may have broader implications for understanding and treating other age-related conditions.
Clinical Translation: Translating findings from basic research on SIRT1 into clinical practice holds promise for improving the management and outcomes of AMD patients. Clinical trials investigating the efficacy and safety of SIRT1-targeted interventions in AMD are warranted to validate the therapeutic potential of SIRT1 modulation in a clinical setting.
Overall, the study on the dual role of SIRT1 in retinal development and its therapeutic implications for AMD contributes to our understanding of retinal biology and holds promise for the development of innovative treatments for AMD and other age-related retinal diseases.
The study aimed to elucidate SIRT1's role in both regulating retinal development and serving as a potential therapeutic target in age-related macular degeneration, shedding light on fundamental biological processes underlying retinal health and offering insights into innovative treatment approaches for AMD. By understanding how SIRT1 influences retinal development and contributes to AMD pathology, we seek to develop targeted interventions that could mitigate disease progression and preserve vision in affected individuals.
Eyes were obtained from the Eye Bank of Ispahani Islamia Institute and Eye Hospital, Bangladesh (Dhaka, Bangladesh), placed in 10% formalin, processed and embedded in paraffin for sectioning. Three eyes were obtained from non-life-threatening injuries as controls and nine eyes were obtained from cadavers and used for immunohistochemical analysis. All eyes met the necessary criteria established for the inclusion of eyes in the control and experimental groups, namely, the absence of documented ocular disease on fundus examination and death with concomitant ocular trauma or sepsis. Tissue from nine eyes was used for the analysis of AMD features: three female cadavers (aged 17, 53 and 90 years) and six male cadavers (aged 58, 62, 71 and 79, 81, 87 years). In addition, sections from 33 eyes of human embryos at 6 (13) and fetuses at 12 (12), 18 (5) and 38 (3) weeks were examined and analyzed by immunohistochemistry. Eyes from 15 outbred mice were used to study the developing retina, since the mouse retina at this stage is not fully mature and is known to contain immature retinal progenitor cells. All eyes were placed in 10% formalin for 48 hours before processing and paraffin embedding.
To understand to what extent SIRT1 was expressed in the retina, we immunostained a number of tissues, which enabled us to derive generalized conclusions. Given our interest in SIRT1 in development, we stained eye specimens from donors of various ages, including eyes from fetuses, young adults, and elderly adults. Additionally, we examined the expression of the innate SIRT1 inhibitor, DBC1, in the same specimens. Of particular interest to us was whether or not SIRT1 and DBC1 were co-localized. The immunohistochemistry results demonstrated that SIRT1 was exclusively cytoplasmic in all human eyes studied and DBC1 was exclusively nuclear, with the exception of the ganglion cell layer where it was both nuclear and cytoplasmic. Importantly, all retinal specimens stained positive for both SIRT1 and DBC1.
Given that our in vitro work with SIRT1 would entail the use of mouse retinal progenitor cells, we sought to determine whether or not the expression of SIRT1, and its inhibitor DBC1, was similar in mouse retinas and human retinas. Accordingly, we immunostained eyes from mice of various ages (age range: P1-P347) with the same two antibodies (which react with both mouse and human tissue) to see if the staining pattern was the same. For SIRT1, strong cytoplasmic staining was seen in the IPL, OPL and photoreceptor inner segments in all eyes. The ONL was consistently negative while weak, sparse positivity was seen in the INL in some eyes (n= 8). The GCL was consistently positive in all eyes (strong) and cytoplasmic, with two eyes showing very weak nuclear staining in addition to cytoplasmic positivity. No statistically significant correlations existed between nuclear localization and age. P1 eyes had cytoplasmic staining only.
DBC1 was strongly expressed in the GCL layer (nuclear) and INL, while moderately expressed in the ONL in all eyes. This pattern was consistent in all eyes, including P1 mouse eyes. Of note, this was also the same pattern seen for DBC1 in human eyes.
Before studying SIRT1 in mRPCs, we sought to further characterize these cells. Our objective was to isolate and re-culture sub-populations of cells based on s ize. This was achieved by using FACS. A panel of antibodies was used to initially characterize the three subpopulations of mRPCs obtained from FACS. Figure 1 lists the antibodies used for each subpopulation. The expression of each of the markers assessed was given a grade of negative (-), <50% of cells positive (+), or >50% of cells positive (++) (Figure 1).
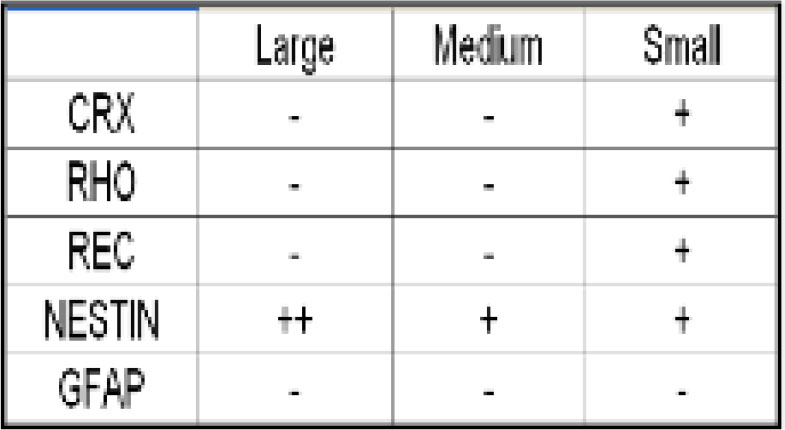
Figure 1. Results from staining populations of mRPCs isolated based on size using FACS. Cells classified as Medium and Large had similar immunocytochemical profiles based on the markers studied.
Small mRPCs had a unique immunocytochemical profile. Importantly, small cells expressed markers of photoreceptor precursors. All cells were negative for the glial cell marker, GFAP, most likely because this marker is most commonly expressed in partially and fully differentiated retinal progenitors. Our results revealed that SIRT1 did not shuttle to the nucleus and was thus expressed in the cytoplasm exclusively at all time points assayed. This result was consistent for cells from both passage 9 and passage 17, suggesting that the shuttling observed in neural progenitors may not happen in retinal progenitors of mouse origin. Results demonstrated that SIRT1 expression could be detected in both the nucleus and cytoplasm of hRPCs as a population. As a final step in assessing the role of SIRT1 in retinal development, we downregulated SIRT1 expression via siRNA and evaluated key retinal development genes. A pool of four different siRNAs targeting SIRT1 was used to achieve an acceptable level of SIRT1 silencing.To quantify the decrease in SIRT1 expression, comparisons were made between SIRT1 siRNA-transfected cells and AllStars negative control siRNA-transfected cells. Also illustrated is the relative change in SIRT1 expression between the untransfected control condition and the negative control siRNA-transfected condition. No significant difference was seen in the latter case.
The first step in determining if SIRT1 represented a possible target in neovascular AMD was to evaluate its expression in human choroidal neovascular membranes. This expression was then compared with expression in the corresponding normal tissues in adult donor eyes. Nine CNV membranes were immunostained and seven were found to be immunopositive for SIRT1 expression. Only seven of the nine membranes had sufficient RPE cells for evaluation, of which 4 w ere SIRT1 positive. Seven of nine membranes had expression of SIRT1 in vascular endothelial cells. Notably, the 2 negative sections were also negative in the other cell types assessed. Normal human eyes had a lower incidence of positivity in the RPE and vascular endothelial cells than CNV membranes, though the small sample size prevented us from drawing conclusions with statistical significance. Nevertheless, these data suggest that an increase in SIRT1 expression in both RPE cells and endothelial cells may be associated with the occurrence of CNV (Figure 2, 3).
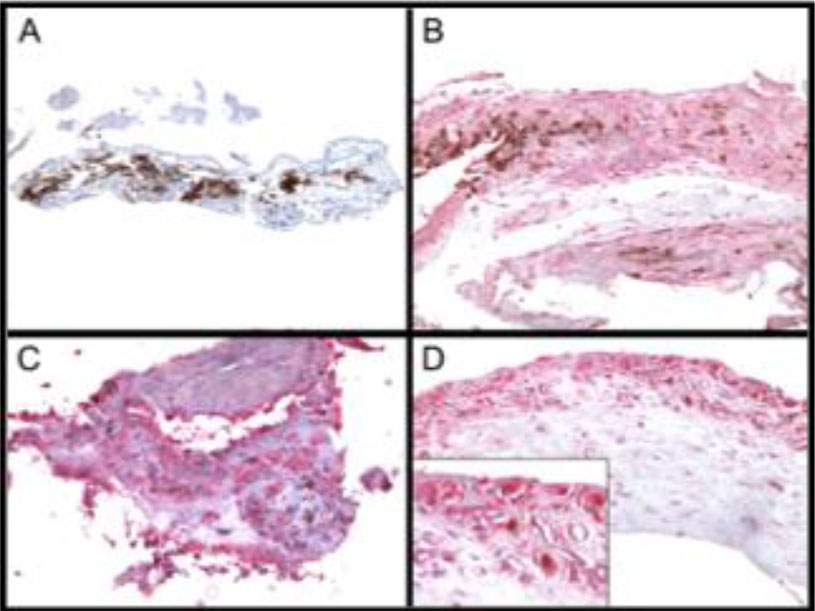
Figure 2. SIRT1 expression in choroidal neovascular membranes. A) Negative. B) Weak expression. C) Strong expression. D) Insert shows vascular endothelial cell staining.
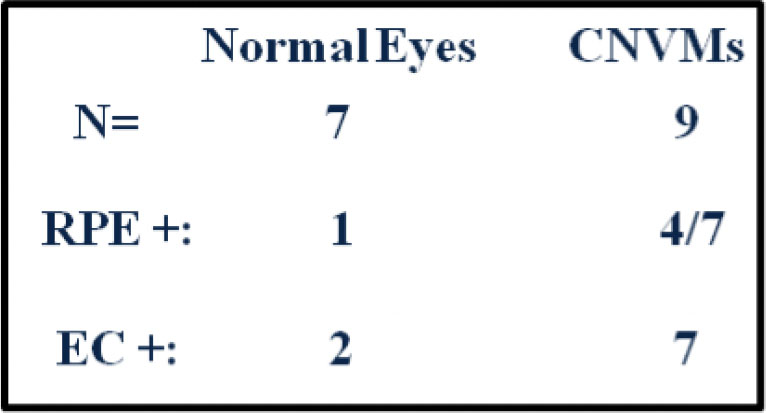
Figure 3. Comparison of SIRT1 expression in normal eyes and CNV membranes. SIRT1 staining was more commonly seen in RPE and endothelial cells (EC) in CNV membranes than in normal eyes.
We next aimed to further investigate the possible relationship between SIRT1 expression and AMD. Since RPE cells in vivo are a possible source of pro-angiogenic cytokines during CNV formation, and because of the availability of a well-characterized RPE cell line (ARPE-19), we chose to further explore this relationship. We looked at expression of SIRT1 in ARPE-19 cells using confocal microscopy. Results suggested that SIRT1 is expressed in ARPE-19 cells predominantly, if not exclusively, in the cytoplasm. These results set the stage for further studies with the well-known SIRT1 inhibitor, nicotinamide (NIC).
We next assessed the cytotoxic effects of the various treatment conditions on cultured ARPE-19 cells using Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) to ensure that the observed changes in cytokine secretion were not due to changes in cell viability.
Results demonstrated that the highest level of cytotoxicity was seen in the NIC+CoCl2 condition (3.88% +/- 0.60% TUNEL positive cells). This amount of cell death is insufficient to explain the observed changes in cytokine secretion, suggesting that these changes can be attributed to the various treatment conditions.
The data presented in this study have provided support for SIRT1 as a regulator of retinal development. Its expression is seen throughout developing and adult retinas as well as in progenitor cells in culture. The exact relationship between subcellular distribution of this protein and retinal development remains to be determined. More importantly, however, we have shown that expression of critical transcription factors that regulate retinal development depend on S IRT1 expression. These insights will be the foundation for additional studies with retinal progenitors. Future studies aimed at optimizing yield of photoreceptor precursors for eventual transplantation should consider the importance of SIRT1 in transcription of key photoreceptor genes.
The findings outlined in this study for the SIRT1 inhibitor, Nicotinamide, highlight this agent as a putative regulator of hypoxic and angiogenic signaling in retinal pigment epithelial cells. The SIRT1 expression seen in choroidal neovascular membranes suggests that the activity of Nicotinamide may be partially mediated by inhibition of SIRT1 activity. Future studies should evaluate the efficacy of this drug in animal models of age-related macular degeneration. Additionally, studies should be performed to evaluate secretion of other pro-inflammatory and pro-angiogenic cytokines from RPE cells following treatment with Nicotinamide. These studies should also address the effects of the drug on the expression of various receptors on vascular endothelial cells.