- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 4. DOI 10.35630/2024/14/4.417
The most common type of alopecia is androgenetic alopecia. It is estimated to affect about 80% of Caucasian men and about 42% of women. It is a serious problem that significantly reduces patients' quality of life. The second most common type is telogen alopecia. Also included in the study is alopecia areata, which affects up to 2% of the general population. Trichoscopy is a quick and precise diagnostic method for these types of alopecia. During a trichoscopic examination, we can visualize characteristic structures such as the perifollicular epidermis, hair follicle openings, and hair shafts. Using trichoscopy, it is possible to quickly determine the severity of hair loss and diagnose specific types of alopecia. Additionally, trichoscopy thoroughly allows for monitoring the response to treatment for various types of hair loss. The trichoscopic characteristics of different types of alopecia make it possible to identify the type of alopecia and choose the appropriate therapy. At the same time, other diagnostic methods take more time and are not as accurate in determining the problem.
The aim of this work: This study aims to determine the usefulness and role of trichoscopy in the diagnosis and monitoring of non-scarring alopecia. Also, to explain the benefits and limitations of this diagnostic method and compare it to other methods. As a result, clinicians need to use this method as one of the first to diagnose non-scarring alopecia to begin appropriate treatment for a patient struggling with this problem as soon as possible.
Methods: The research method was a thorough, critical analysis of scientific articles in the Google Schoolar and Pubmed databases. Content from 2012-2024 was reviewed however, most of the studies cited were published in 2018 or later. We focused on the characteristics of non-scarring alopecia, its trichoscopy features, and diagnostic methods.
Conclusions: Trichoscopy is a simple, non-invasive method that may help diagnose various diseases with non-scaring alopecia. This method is painless, precise, and faster than others to diagnose and monitor non-scarring alopecia.
Keywords: alopecia; nonscarring alopecia; trichoscopy; diagnostic
Trichoscopy is a noninvasive and simple examination that can be performed in outpatient conditions using a digital or handheld dermatoscope. This diagnostic tool provides valuable information about hair loss-related disorders through visualization and identification of characteristic signs and structures. Trichoscopy examines the morphology of structures that are not visible to the naked eye, including intrafollicular and perifollicular features, as well as changes in the thickness and form of the hair shaft. Hair loss is a common issue among dermatological patients, affecting both men and women of various ages. The epidemiology of alopecia depends on the type of alopecia, the most common type of alopecia is androgenetic alopecia. It is estimated to affect about 80% of Caucasian men and about 42% of women [6]. It is a serious problem that significantly reduces patients' quality of life. The second most common type is telogen alopecia. Also included in the study is alopecia areata, which affects up to 2% of the general population [21]. In this article we explained the methodology of trichoscopy, the most common types of non-scarring alopecia and their trichoscopic features, enabling the establishment of a proper diagnosis, identification of the stage and extent of hair loss, monitoring the effects of therapy with the aid of trichoscopy, also benefits and limitations of this diagnostic method and we compared this method with other diagnostic methods of alopecia [10].
The research method was a thorough, critical analysis of scientific articles in the Google Schoolar and Pubmed databases. Content from 2012-2024 was reviewed however, most of the studies cited were published in 2018 or later. We focused on the characteristics of non-scarring alopecia, its trichoscopy features, and diagnostic methods.
This study aims to determine the usefulness and role of trichoscopy in the diagnosis and monitoring of non-scarring alopecia. Also, we mention the benefits and limitations of this diagnostic method and compare it to other methods. As a result, clinicians need to use this method as one of the first to diagnose non-scarring alopecia to begin appropriate treatment for a patient struggling with this problem as soon as possible to improve the quality of life of these patients. Our research objective was also to present the most common types of non-scarring alopecia and their specific trichoscopic features, to facilitate the use of this diagnostic method.
Trichoscopy examination is performed using any handheld dermoscope. Trichoscopy can also be performed with digital dermoscopes – videodermoscopes. The advantage of these devices is easier photographing and the ability to achieve greater magnification of the image; unfortunately, they are more expensive and time-consuming as a result. We distinguish dermoscopes that require immersion fluid and those that, through polarized light, eliminate reflections from the stratum corneum. Polarized light devices can have a noncontact or contact lens. Hybrid dermoscopes are also used, which have both contact and non-contact attributes. The choice of a specific dermoscope is a matter of individual preferences. Most handheld dermoscopes use a 10-fold magnification, whereas digital dermoscopes allow observation of the skin surface with a working magnificatiVB54Oon from 20 to 100-fold and higher. Lower magnifications are used for examining a large area of the scalp, while higher magnifications are useful for visualizing fine details. Unlike dermoscopy for skin cancer, trichoscopy differs, among other things, in the large area that needs to be examined. For example, when diagnosing diffuse hair loss, the occipital and frontal areas should be examined. In the case of focal alopecia, the hairless area and the hair-hearing margin should be investigated. Sometimes, in the case of certain diseases, an additional evaluation of the eyebrows can be used [22, 25].
Washing hair mostly does not affect trichoscopy results. Hair colorization also does not influence the examination outcome; it may even aid in analyzing hair shaft structure and assessing hair thickness (in individuals with gray or light blond hair) [25]. Trichoscopy may pose difficulties in cases of scalp discolorations and buildup [18].
With trichoscopic examination, it is possible to visualize structures including hair shafts, perifollicular epidermis, skin microvasculature and hair follicle openings [22]. The examination should be started without the use of immersion fluid, as some trichoscopic features are visible with its use (vascular component), while others are visible without it (exfoliation). The choice of trichoscopic examination technique depends on the type of alopecia. For example, in cases of focal alopecia, the center of the lesion is primarily analyzed to assess the presence of hair follicles, aiming for a differential diagnosis between cicatricial and non-cicatricial alopecia. Additionally, in this type of alopecia, it is important to examine the edge of the lesion. In the diagnosis of diffuse alopecia, it is essential to differentiate between androgenetic alopecia and telogen effluvium - the frontal and occipital regions should be separated and attention should be paid to differences in hair thickness, the proportion of hair units with 1, 2, and 3 shafts, as well as the number of yellow dots. If such differences are present, this favors a diagnosis of androgenetic alopecia. In the absence of differences, further diagnostic tests such as laboratory investigations and trichogram should be conducted [18].
The key criterion for classifying alopecia is the presence of scarring, which divides it into two main categories: scarring alopecia and non-scarring alopecia. Among these two forms, non-scarring alopecia is significantly more common. It results from changes in the natural hair growth cycle, follicular miniaturization, hair fragility, or a combination of these factors. The primary characteristic of non-scarring alopecia is the preservation of follicular integrity despite hair loss. The causes of this type of alopecia can include various factors such as stress, nutritional deficiencies, hormonal imbalances, general health issues, medications, infections, genetic factors, and mechanical damage due to improper hair care [26].
The most known types of non-scarring alopecia include alopecia areata, androgenetic alopecia, female pattern hair loss, anagen effluvium, short anagen syndrome, loose anagen syndrome, telogen effluvium, trichotillomania, traction alopecia, tinea capitis, and congenital triangular alopecia [26]. In this article, we focus on the three most common types of non-scarring alopecia and their trichoscopic features.
Androgenetic alopecia (AGA) is a chronic condition characterized by the gradual miniaturization of hair follicles in the frontal and parietal areas, leading to the loss of the follicles' ability to produce hair. It is the most common type of alopecia diagnosed in both men and women, with a progressive course, and the clinical presentation may vary depending on the gender [8, 9, 35].
The condition can appear at any age, even before puberty, with the risk increasing with age. It is estimated to affect about 80% of Caucasian men and about 42% of women [8, 13]. In addition to the aesthetic aspect, androgenetic alopecia can lead to stress and depression, significantly reducing the quality of life for those affected [8].
Scientific evidence indicates a multifactorial pathogenesis of androgenetic alopecia [8, 35]. Key factors in the development of this condition include genetic predisposition, the action of androgens on hair follicles, and microinflammation around the hair follicles. In individuals with a genetic susceptibility, environmental factors such as UV radiation exposure, pollution, contact with irritating chemicals, and disturbances in the scalp microbiome, as well as lifestyle factors like smoking, can further stimulate the production of free radicals, which may exacerbate the symptoms of the disease [8, 32].
Trichoscopy findings are the variability of the hair shaft and dominance of follicles with a single hair [10]. In the frontal region, the coexistence of hairs of different thicknesses is observed (the so-called heterogeneity of hair shaft thickness; more than 20% of hairs have reduced thickness). In addition, an increased percentage of mesquite hairs (more than 10%), more hair units with a single stalk, and fewer units with three stalks are found compared to the occipital region [6].
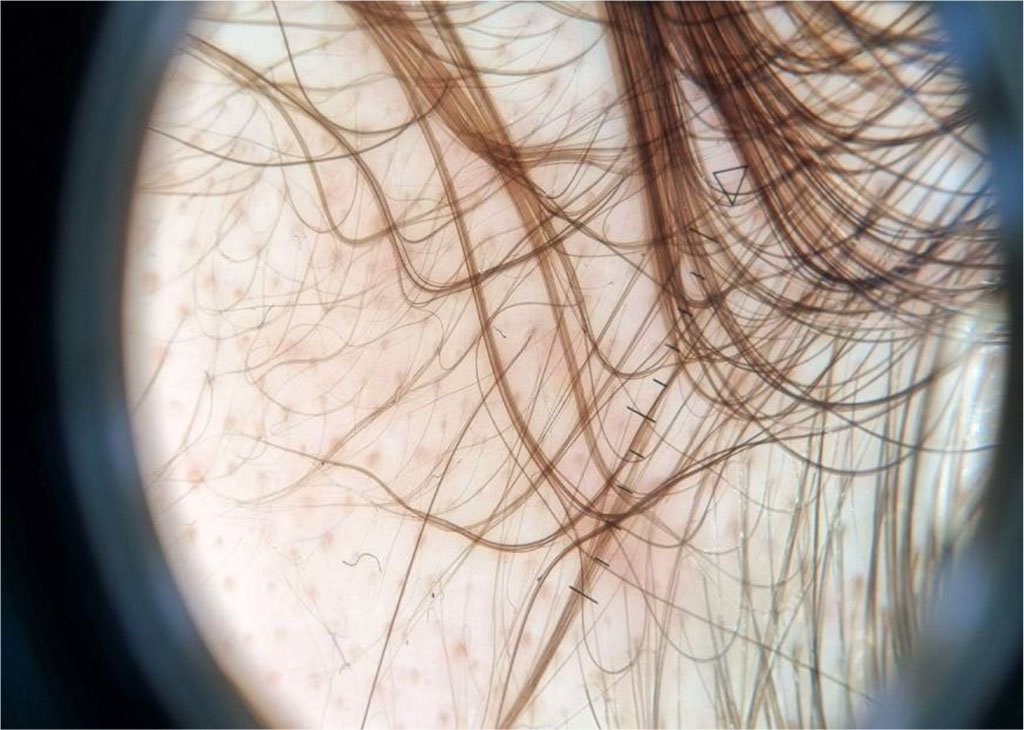
Figure 1: Sample trichoscopic image of a patient with androgenetic alopecia
It's a common chronic autoimmune disease characterized by hair loss, affecting up to 2% of the general population [37]. It can occur at any age, with similar frequencies in both genders [26]. In alopecia areata, hair follicles are damaged due to infiltration by T lymphocytes [19, 37]. Genetic and environmental factors also contribute to the pathogenesis of this condition [37]. Although psychological stress is considered a major pathogenetic factor in alopecia areata, infants and newborns also suffer from this disease, suggesting the presence of other environmental factors [15].
Trichoscopy findings are exclamation point hairs, yellow dots, hypopigmented vellus hair, groupings of short vellus hair, monilexrith-like hair distribution, angled hair [10]. Another symptom seen in trichoscopy is the presence of mesquite hairs, which are less than 3 mm long and less than 0.03 mm thick and lack pigment. Hair regrowth manifests mainly as new anagen hairs growing straight and round hairs that are regularly curled at their tip [21].
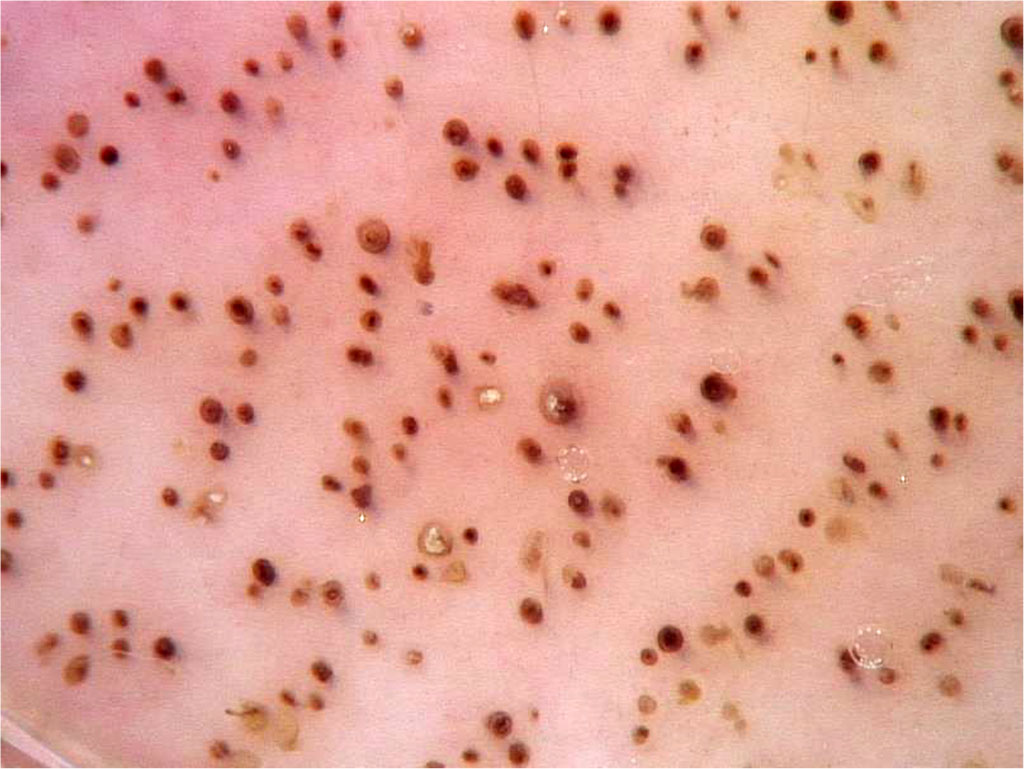
Figure 2: Sample trichoscopic image of a patient with alopecia areata
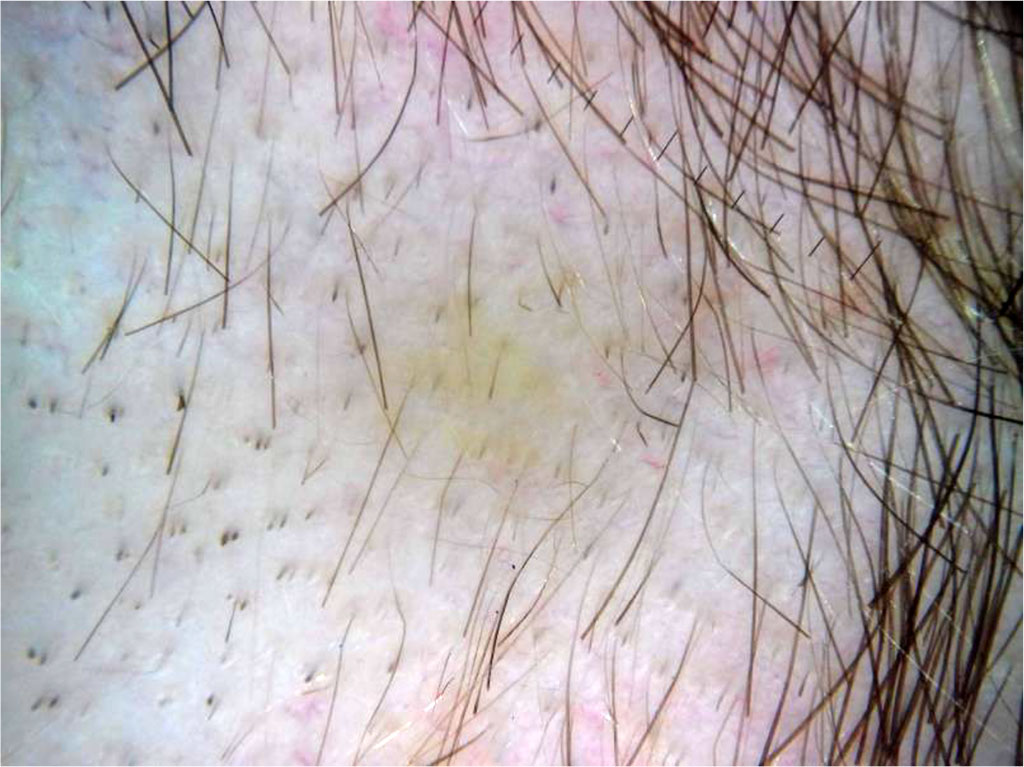
Figure 3: Yellow dots characteristic of alopecia areata as visualized through trichoscopic examination
Telogen effluvium (telogen alopecia) is the second most common type of non-scarring alopecia which affects both women and men. It is a type of hair loss characterized by a shortened anagen (active hair growth) phase, leading to an increase in the number of hairs transitioning into the telogen (resting) phase, resulting in shedding. There is typically a latency period of about 3 months between the onset of hair loss symptoms and the causative factor. Noticeably increased shedding occurs around 8 weeks after the causative factor and usually lasts for 3 months before spontaneously resolving, unless there are other triggering factors [28, 36].
The most common causes of excessive hair shedding in telogen effluvium include stress, high fever, medications, general anesthesia, heavy metal poisoning, hormonal imbalances, intense weight loss, and dietary changes [36]. Symptoms include increased hair shedding during washing and combing. The pull test, which reveals a positive result, is helpful in diagnosis.
Trichoscopic examination may show a decrease in hair follicle yield [36]. Trichoscopy findings are also follicles with a single hair and regrowing hairs [10].
Trichoscopy can help assess the severity of hair loss. In the case of alopecia areata, there are two scales: the SALT (Severity of Alopecia Tool) scale and the AAS (Alopecia Areata Score) scale, which partially relies on the SALT scale. The SALT score provides information about the scalp's surface area affected by hair loss. This method is presented as follows: S0 - no hair loss; S1 - less than 25% hair loss; S2 - 26−50% hair loss; S3 - 51−75% hair loss; S4 - 76−99% hair loss; and S5 - 100% hair loss (alopecia totalis). Moreover, trichoscopy results can serve as markers of disease severity. According to some studies, black dots, yellow dots, broken hairs, and tapering hairs have been found to correlate with the degree of severity positively [2].
Trichoscopy is a sensitive method used to monitor the response to treatment for various types of hair loss [12].. To determine whether a particular medication is effective, at least 12 weeks of observation is necessary [34]. In treating alopecia areata, the growth of short vellus hairs, tapering hairs, broken hairs, and black dots indicates that patients have responded effectively to treatment, while yellow dots are the least responsive to treatment [2]. In the case of androgenetic alopecia, the effectiveness of therapy is indicated by an increase in the number of terminal hairs, a reduction in the number of vellus hairs, and an increase in hair density[12].
Trichoscopy provides characteristic features specific to different types of hair loss (known as trichoscopic features). These features allow for the preliminary diagnosis of the type of alopecia and the initiation of appropriately tailored therapy [2]. For example, after diagnosing androgenetic alopecia, treatment with minoxidil and/or finasteride (or spironolactone for women) should be started. If perifollicular hyperpigmentation is observed in trichoscopy, additional treatment with topical clobetasol should be introduced. Follow-up trichoscopy should be performed every 6 months. If improvement is noted, the treatment should be continued, while in case of deterioration, modification of the treatment is necessary. If stabilization occurs in two consecutive examinations, one medication can be discontinued, the dosage reduced, or the treatment stopped entirely [6].
Diagnosis of alopecia includes methods such as clinical examination, trichoscopy, histopathological examination of a scalp section, laboratory tests, trichogram, daily hair loss, wash test, pull test, hair weighing, light microscopy, scanning electron microscopy (SEM) and transmission electron microscopy (TEM), and phototrichogram [3, 5, 24].
A trichogram is a diagnostic test that involves microscopic evaluation of about 100 of a patient's hair follicles, which are taken by the doctor. The hairs are usually taken in equal amounts from two areas of the scalp, one of which is the frontal area. The hairs are taken in one sure motion with tweezers placed about 0.5 cm from the surface of the skin. The result is usually presented as the percentage of hair that is in each phase of the cycle. The trichogram is most diagnostically useful in diagnosing acute telogenetic alopecia, but in the case of androgenetic alopecia, the results of this test are inconclusive. Thus, it is not a universal diagnostic method and usually requires further testing [24].
Daily hair loss, wash test, and pull test or hair weighing are based on observation of hair loss. They are not sufficient methods to make a diagnosis and determine the exact type of baldness. It is also worth noting that some diseases leading to baldness are not associated with increased hair loss. An example is androgenetic alopecia, in which the hair follicles gradually miniaturize, and the hair becomes thinner and shorter, and there may be no obvious increase in terminal hair loss. They have a high error rate and only indicate the presence of a hair loss problem, but do not determine the exact cause, so they cannot be independent diagnostic methods [3, 17].
The method of classical light microscopy is used to evaluate hair shafts. Usually several to dozens of hairs are taken for examination. It is worth noting, however, that in the case of some conditions, a much larger number of hairs may need to be taken to find a single hair with a characteristic abnormality to make a proper diagnosis. This method is also subject to a high rate of false-negative results [24].
Both methods, SEM and TEM, are not commonly used in everyday trichology practice. Only a small section of a single hair is evaluated during a single examination, which means that the results may not be very representative. An additional problem is the risk of damaging the hair both during sample preparation and during the examination itself. SEM and TEM are sometimes used as complementary methods to help visualize or more accurately identify changes detected by other techniques [24].
A phototrichogram is a non-invasive test that involves taking macro photographs of the scalp. After shaving a section of skin, the first photo is taken, and then, after about 72 hours, the photograph is repeated. Hair in the anagen phase will elongate by about 1 mm, while hair in the telogen phase will be practically invisible, only the follicle mouths will be visible. On this basis, the ratio of anagen to telogen hair can be calculated. This is a method that takes much longer than a trichoscope examination and covers a specific, small area of the scalp. It also requires shaving a particular section of the scalp, which does not need to be done with trichoscopy [3, 30].
It should be mentioned that a well-performed trichoscopy is a sufficient method to diagnose a given type of non-scarring alopecia and to exclude other causes. It does not require hair harvesting, and for some patients, harvesting hair for examination can be a hassle to accept. Each of the listed types of non-scarring alopecia has its own characteristic trichoscopic features, so this method is universal for each type. It is also possible to examine the entire scalp surface in a single examination, rather than being limited to one part of the scalp, as with the other methods mentioned above. The trichogram, on the other hand, has no diagnostic significance in the diagnosis of alopecia areata, for example, so it is less universal than trichoscopy [21].
When in doubt, histopathological diagnosis of a scalp section is included. It is the most invasive diagnostic method for alopecia. It involves taking two sections from areas where baldness is present for histopathological examination under a microscope. The patient waits several weeks for the results of this examination. This significantly postpones the start of treatment for a given patient and often causes a lot of stress for patients. This method is often used, but for the diagnosis of scarring alopecia [3, 23]. Some types of alopecia can be diagnosed based on a well-gathered medical history and clinical assessment, as in the case of androgenetic alopecia in men, but in women - these methods should be supplemented with trichoscopic examination, and in cases of suspicion during trichoscopic examination of coexisting telogen alopecia - laboratory tests and trichogram. Thus, trichoscopy is a method that allows you to quickly and accurately confirm a particular type of alopecia based on changes in the trichoscopic image and carry out a differential diagnosis with other types of alopecia, all during a single medical visit [6].
Laboratory tests in the diagnosis of alopecia are a non-specific method and it is not possible to diagnose the type of non-scarring alopecia based on their results alone.
Knowing the trichoscopic features of a particular type of non-scarring alopecia, clinicians are able to carry out the diagnostic process very quickly, make a diagnosis, and incorporate treatment of the disease entity at the same medical visit. This article shows the differences in the trichoscopic images of the most common types of non-scarring alopecia, so that the differential diagnosis will be systematized, and the patient will avoid long, sometimes unnecessary additional diagnostics.
Benefits
As previously mentioned trichoscopy is a simple and noninvasive method. It is an accurate, painless, and faster method than those mentioned above. One of its biggest benefits is the possibility of assessing the structure of the hair shaft without the need to collect hair for examination using light microscopy. It allows examining the scalp for hair with abnormal structure and enables diagnosis of most genodermatoses accompanied by alopecia, such as pilli annulate, monilethrix, and trichorrhexis nodosa [16]. Moreover, trichoscopy allows for the differentiation of androgenic alopecia in women with chronic telogen effluvium or trichotillomania and other nonscarring types of alopecia. More details are available on the Types of alopecia and their trichoscopic features. In a study Assessment, the reliability, and validity of trichoscopy in the evaluation of alopecia in women from 2021 researchers concluded that trichoscopy can help reach a definitive diagnosis in doubtful cases. Out of 200 participants, 57.5% (n=115) were diagnosed with female pattern hair loss (FPHL) using trichoscopy [27].
Limitations
Since trichoscopy is performed with a handheld video dermoscopy or at dermoscopy, it is crucial to remember that images depend on the immersion medium used and the type of resolution used by a dermoscope. Fernandez et al. suggested using an ultrasound gel as an immersion fluid in contact dermoscopy [10]. It is important to correctly interpret or recognize structures in trichoscopy, otherwise, it may lead to decreased diagnostic accuracy. However, in some cases, images of different diseases can be similar. Because of that, it is crucial to put it in the clinical context [10, 22].
Directions of development of research on trichoscopy
Because of the lockdown caused by the pandemic of COVID-19, the demand for remote alternatives has increased. To solve this problem Abraham et al. suggested a “linear trichoscopy” process. A smartphone in the panorama is moved linearly, taking pictures of an area of interest, with a dermoscope attached to it [1, 14]. Using trichoscopy in alopecia areata (AA) is widely described in the dermatological literature, yet studies on this method in beard alopecia areata (BAA) are limited. In 2019 Bhandary et al. suggested that further studies on dermoscopy in BAA may concede newer, nonfollicular findings, in addition to follicular already reported in AA. Their academic work describes uncommon findings such as tulip hair, perifollicular hemorrhage, i-hair, and peripilar sign [4]. In the academic review ”Trichoscopy-Derived Hairline Recession Equivalent in Monitoring Frontal Fibrosing Alopecia” researchers found two hair loss patterns: 1) diffuse loss within the width of 10mm, 2) significant decrease of density within the width of 1mm. They concluded that the density profile of hair can be used to characterize different disease progression patterns [29].
Potential applications of trichoscopy in other areas of dermatology
In the differential diagnosis of types of alopecia, alopecia caused by secondary syphilis should be taken into account- syphilitic alopecia (SA). The main difference between SA and areata alopecia found in trichoscopy is the absence of an exclamation mark in SA. However, more research is needed on this topic [7, 20, 31]. In trichoscopy, specific morphological features have been identified as highly indicative of various dermatological conditions, providing valuable diagnostic insights. The following is a summary of these features observed in different skin diseases: contact dermatitis (twisted red loops), dermatomyositis (lake-like vascular structures), discoid lupus erythematosus (follicular plugs, erythema encircling follicles), lichen planopilaris (milky red areas or fibrotic patches), pemphigus foliaceus (white polygonal structures, serpentine vessels), pemphigus vulgaris (red dots with whitish halo, lace-like vessels), psoriasis (diffuse scaling, simple and twisted red loops, red dots and globules, glomerular vessels), seborrheic dermatitis (atypical vessels, thin arborizing vessels, structureless red areas), tinea capitis (comma-shaped hairs, corkscrew hairs, morse code-like hairs, zigzag hairs, bent hairs, block hairs, I-hairs). Trichoscopy may also help in differentiating between Trichopython and Microsporum tinea capitis [11, 33].
Trichoscopy is a beneficial diagnostic procedure in dermatology, especially in diagnosing alopecia. Trichoscopy provides us with valuable information about the severity of hair loss. Each type of non-scarring alopecia has different trichoscopic features, thanks to which they can be distinguished from each other, which leads to accurate diagnosis and appropriate treatment. It also allows for monitoring the response to treatment. Trichoscopy is a dynamically evolving diagnostic tool, as indicated by the possibility of performing it using a phone and the development of beard trichoscopy. It is accurate, painless, and faster than other methods, allowing the doctor to make a diagnosis in just one medical visit, while other methods of diagnosing alopecia take several days or even weeks in clinical practice. The information presented in the above article confirms that trichoscopy should form the basis of diagnosing alopecia, therefore it is important to increase dermatologists' knowledge about its significant impact on the diagnostic process.
All authors have read and agreed with the published version of the manuscript.
Author Contributions: KM: conceptualization, literature review, writing - original draft preparation; HD, SS and ŁS: literature review, writing - review and editing.
The authors deny any conflict of interest
The study received no specific funding