- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 3. DOI 10.35630/2025/15/3.309
Background: Glucagon-like peptide-1 receptor agonists are a class of hypoglycemic drugs primarily used in the treatment of type 2 diabetes. They mimic the action of endogenous glucagon-like peptide 1 while exhibiting prolonged effects and resistance to degradation by the enzyme dipeptidyl peptidase 4. However, researchers are interested in this drug not only for its incretin properties but also for a range of associated effects. It turns out that it may also be useful in the treatment of chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma.
Aims: The aim of this paper is to review and analyze current scientific reports on the impact of GLP-1 receptor agonists on the progression, symptoms, and pathophysiological mechanisms of these diseases.
Results: Positive effects of GLP-1 agonists may result from reduced COPD exacerbation risk, improved pulmonary function test results, and weight loss in patients receiving GLP-1 analogs.
The findings also suggest that GLP-1 agonists may possess immunomodulatory and anti-inflammatory effects in asthma, opening new therapeutic possibilities, particularly for patients with obesity and chronic inflammatory respiratory diseases.
Conclusion: A summary of the available evidence suggests that GLP-1RA may represent a promising addition to asthma and COPD therapy, yet further research is needed to confirm their efficacy and safety in these indications.
A narrative literature review was conducted using the PubMed and Google Scholar databases were searched. The search included phrases such as “glucagon-like peptide-1 receptor agonists and chronic obstructive pulmonary disease,” “glucagon-like peptide-1 receptor agonists and asthma,” “glucagon-like peptide-1 receptor agonists and COPD,” “GLP-1 and chronic obstructive pulmonary disease,” and “GLP-1 and asthma.” The article is based on publications released between February 2020 and June 2025 that are available in English.
Keywords: Glucagon-Like Peptide-1 Receptor Agonists, chronic obstructive pulmonary disease, COPD, asthma, GLP-1 receptors,
Glucagon-like peptide-1 (GLP-1) is a peptide hormone secreted by intestinal L endocrine cells in response to food intake [15]. One of the most important functions of GLP-1 is its incretin effect, meaning it enhances insulin secretion. Additionally, under conditions of elevated blood glucose levels, GLP-1 inhibits glucagon secretion. This hormone also slows gastric emptying, delaying digestion and glucose absorption into the bloodstream. As a result, it mitigates the sharp postprandial rise in blood glucose levels. Slowed gastric emptying also promotes earlier satiety, potentially leading to a reduced overall food intake [7], [15]. GLP-1 binds to its specific receptor (GLP1R), which is found not only on pancreatic β cells but also in the heart, lungs, kidneys, central and peripheral nervous system, adipose tissue, adrenal glands, eyes, bones, and other parts of the gastrointestinal tract [24]. The discovery of the aforementioned effects of GLP-1 paved the way for its use in hypoglycemic treatment. Unlike endogenous GLP-1, GLP-1 receptor agonists (GLP1-RA) have a longer half-life and are resistant to dipeptidyl peptidase-4. The main GLP1-RA include liraglutide, exenatide, and dulaglutide.
Asthma and chronic obstructive pulmonary disease (COPD) are among the most common chronic respiratory diseases, significantly burdening healthcare systems and diminishing patients' quality of life [8]. Despite the availability of numerous therapeutic options, many patients fail to achieve full symptom control, prompting the search for new, more effective treatment strategies. This paper provides a review of the latest research on the potential role of GLP-1 receptor agonists in asthma and COPD therapy, with a particular focus on the mechanisms of action of these drugs and the findings of preclinical and clinical studies.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that plays a key role in blood glucose regulation by stimulating insulin secretion and inhibiting glucagon release. GLP-1 receptor (GLP-1R) agonists are primarily used in the treatment of type 2 diabetes and obesity, however increasing research points to their potential impact on the respiratory system. [6] The first drug in this group was exenatide, which was approved for use in 2005.
Later, additional substances for the treatment of type 2 diabetes were introduced, including liraglutide, dulaglutide, albiglutide, and semaglutide. [38] GLP-1 receptors are present in lung tissue, including alveoli, ciliated cells, smooth muscle, and blood vessels, as well as immune system cells. GLP-1 agonists directly influence immune cell signaling, exhibiting anti-inflammatory properties. This leads to a reduction in eosinophil count, decreased mucus production, limited oxidative stress, and lower levels of pro-inflammatory mediators.
Additionally, GLP-1 agonists promote bronchial dilation, inhibit smooth muscle cell proliferation and migration, and reduce lung fibrosis. Their indirect effects result from weight loss, which improves lung function and mechanics. Spirometry findings show an increase in expiratory reserve volume (ERC), functional residual capacity (FRC), and total lung capacity (TLC). Furthermore, gas exchange efficiency improves, increasing oxygenated blood levels, while respiratory muscles work more efficiently, alleviating breathlessness.
The reduction in adipose tissue decreases the production of inflammatory mediators, further reinforcing anti-inflammatory and antioxidant effects. [18]
Chronic obstructive pulmonary disease (COPD) is one of the most common respiratory illnesses, accounting for over 50% of all chronic respiratory diseases in both sexes in 2017 [33]. Smoking is the main risk factor, but genetics, early-life complications, infections, and harmful environmental exposures also play a role [34]. Common symptoms include chronic cough, sputum production, shortness of breath, and wheezing [25]. A 2010 study found that 18.7% of COPD patients also had type 2 diabetes [12]. Another reported that 19% were diagnosed with diabetes within the past 10 years [14]. People with diabetes also face a higher risk of developing COPD [16]. Shared mechanisms—such as systemic inflammation, oxidative stress, hypoxia, obesity, and high blood sugar—may link the two diseases [20]. GLP-1 receptor agonists (GLP-1RAs), used for diabetes, have anti-inflammatory and antioxidant properties [36], suggesting potential benefits for COPD. Pradhan et al. [29] studied whether GLP-1RAs reduce COPD exacerbations in patients with type 2 diabetes. They included patients who started GLP-1RA therapy (e.g., dulaglutide, exenatide, liraglutide) between 2007 and 2019. The study excluded individuals over 40, prior users of incretin-based therapy, and those with end-stage kidney disease. Participants required one year of medical history and a prior COPD diagnosis in the CPRD database. The primary endpoint was time to first severe COPD exacerbation (ICD-10 codes J41–J44). Secondary endpoints included time to first moderate exacerbation and the number of moderate and severe exacerbations during follow-up. The final cohort included 1,252 patients. Of these, 91.6% had obesity (BMI ≥ 30), 94.3% had a history of smoking or were current smokers, and 62.3% had HbA1c > 8%. The average age was 61.4 years, and mean COPD duration was seven years. GLP-1RA use was linked to a 30% lower risk of severe exacerbations and a 37% lower risk of moderate exacerbations compared to sulfonylureas. GLP-1RAs reduce airway inflammation and hyperreactivity, as shown in mice, human airway samples, and patients with type 2 diabetes and COPD [30],[31],[35]. They also have systemic anti-inflammatory effects, supporting the role of systemic inflammation in COPD. By promoting weight loss, these drugs may also reduce diaphragmatic pressure and improve lung capacity [13],[28].
Despite promising findings, evidence remains limited. More laboratory and observational studies are needed to clarify how GLP-1RAs affect COPD exacerbations. Albogami Y et al. [1] evaluated the association between GLP-1 receptor agonist therapy and the risk of exacerbations of chronic lower respiratory disease (CLRD) in patients with coexisting type 2 diabetes. The CDC and WHO define CLRD as a group of chronic lung diseases, including COPD, chronic bronchitis, emphysema, and asthma, which often coexist or overlap [26]. The authors conducted a retrospective cohort study using data from 2005 to 2017, including individuals aged 17 and older who required hospitalization or outpatient care for type 2 diabetes and CLRD within the year preceding the index date. The index date corresponded to the initiation of GLP-1 receptor agonist or dipeptidyl peptidase-4 inhibitor (DPP-4I) therapy. Patients receiving these therapies as an adjunct to other antidiabetic medications, such as metformin, also met inclusion criteria. The final cohort included 4,150 patients treated with GLP-1 receptor agonists. Compared with patients receiving DPP-4 inhibitors, those treated with GLP-1 receptor agonists experienced fewer hospitalizations and fewer CLRD exacerbations. For the primary endpoint—hospitalization due to CLRD—the incidence rate reached 11 per 1,000 person-years in the GLP-1RA group, compared to 20.6 in the DPP-4I group. For the secondary endpoint—CLRD exacerbation requiring emergency care, outpatient treatment, or systemic glucocorticoid therapy—650 events were recorded. The GLP-1RA group demonstrated approximately 13 fewer exacerbations per 1,000 person-years than the DPP-4I group. These findings suggest that GLP-1 receptor agonists may reduce respiratory complications in patients with type 2 diabetes and CLRD, although randomized clinical trials remain necessary to confirm these observations. Foer et al. [10] conducted a similar retrospective observational study assessing the impact of GLP-1 receptor agonist therapy on COPD exacerbation rates in patients with type 2 diabetes. The study included 1,642 individuals diagnosed with COPD, with data collected between 2012 and 2022. Among them, 328 patients initiated GLP-1RA therapy and formed the treatment cohort. The date of GLP-1RA prescription served as the exposure date, and follow-up continued for six months. The primary outcome measured the total number of moderate and severe COPD exacerbations occurring during the six-month observation period. Results indicated a lower risk of moderate and severe exacerbations in the GLP-1RA group compared to patients treated with sulfonylureas or DPP-4 inhibitors. However, no statistically significant difference in exacerbation rates was found between patients treated with GLP-1 receptor agonists and those receiving sodium-glucose co-transporter-2 (SGLT2) inhibitors. The authors concluded that GLP-1 receptor agonists show potential in reducing COPD exacerbations among patients with type 2 diabetes, but emphasized the need for further studies to establish their role as a preferred therapeutic option in this population.
In 2022, Altintas Dogan AD et al. investigated the effects of liraglutide—a GLP-1 receptor agonist—on lung function in patients with obesity and COPD [2]. The 44-week study enrolled 40 COPD patients aged 40–75 years with a body mass index (BMI) above 27 kg/m² and a smoking history of at least 20 pack-years. Researchers randomly assigned participants to two groups (1:1 ratio): one received 3 mg of liraglutide subcutaneously, while the other received a placebo. Sixteen individuals completed the liraglutide treatment, and 14 completed the placebo regimen. The study assessed outcomes using the COPD Assessment Test (CAT), spirometry, and body plethysmography. It also measured body weight and performance in the six-minute walking test. Evaluations occurred at weeks 4, 20, 40, and 44. Liraglutide consistently induced weight loss throughout the study period. Compared to placebo, the treatment group showed increased forced vital capacity (FVC) and diffusion lung capacity for carbon monoxide (DLCO), along with a reduction in COPD-related symptom burden, as measured by CAT scores. Liraglutide did not improve forced expiratory volume in one second (FEV₁), the FEV₁/FVC ratio, or exercise tolerance assessed by the six-minute walk test. The study also found no significant changes in inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6), or monocyte chemoattractant protein-1 (MCP-1), despite previous reports suggesting their decline during liraglutide therapy [3][23]. Nevertheless, the authors proposed liraglutide as a potential therapeutic option for patients with obesity and COPD and underscored the need for further research to clarify its anti-inflammatory mechanisms. See XY et al. [32] published a 2025 study examining the impact of incretin-based therapies—including GLP-1 analogs—on patients with COPD and type 2 diabetes. The study included individuals undergoing triple combination inhalation therapy, consisting of a long-acting β₂ agonist (LABA), a long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid (ICS) [12]. Researchers conducted a retrospective cohort study using the TriNetX database, which compiles electronic medical records from healthcare institutions. The study enrolled 4,184 adults diagnosed with both COPD and type 2 diabetes and receiving GLP-1 analog therapy. The primary endpoint was the occurrence of COPD exacerbations. Secondary endpoints included pneumonia, acute respiratory distress syndrome (ARDS), intubation, oxygen dependence, and all-cause mortality. The mean follow-up period lasted one year. During the observation period, 382 COPD exacerbations occurred in the GLP-1 analog group. Compared to patients treated with dipeptidyl peptidase-4 inhibitors (DPP-4Is), those receiving GLP-1 analogs experienced lower rates of COPD exacerbations, pneumonia, and oxygen dependence. Additionally, the all-cause mortality rate was 40% lower in the GLP-1 analog group than in the comparator group. The authors attributed these findings to reduced exacerbation risk, improved pulmonary function parameters, and body weight reduction associated with GLP-1 analog therapy.
Table 1. Summary of Studies on the Effects of GLP-1 Receptor Agonists in Patients with COPD and Type 2 Diabetes.
| Authors | Year of study | Main findings |
| Pradhan et al. | 2022 | GLP-1RAs reduced the risk of severe COPD exacerbations by 30% and moderate ones by 37% compared to sulfonylureas. |
| Albogami Y et al. | 2021 | GLP-1RAs lowered hospitalizations and exacerbations of chronic lower respiratory disease (CLRD) compared to DPP-4 inhibitors. There were 13 fewer exacerbations per 1,000 person-years. Further clinical trials are needed. |
| Foer D et al. | 2023 | GLP-1RAs reduced moderate and severe COPD exacerbations vs. sulfonylureas/DPP-4Is, but showed no significant difference compared to SGLT2 inhibitors. Data included 1,642 patients, with 328 receiving GLP-1RA therapy. |
| Altintas Dogan AD et al. | 2021 | Liraglutide improved FVC and DLCO and reduced COPD symptoms (CAT score), but did not improve FEV₁, FEV₁/FVC ratio, or exercise tolerance. No significant changes were observed in inflammatory markers. |
| See XY et al. | 2025 | GLP-1RAs reduced COPD exacerbations, pneumonia, oxygen dependence, and all-cause mortality (by 40%) compared to DPP-4Is. |
Asthma is a respiratory disease characterized by inflammation and structural changes in both large and small bronchi [18]. In patients with asthma, complex interactions occur between different types of immune cells, leading to bronchial hyperreactivity, excessive mucus production, and airway narrowing. The most common symptoms of asthma include episodes of wheezing, shortness of breath, and a sensation of chest tightness. Due to its high prevalence, it is estimated that around 300 million people worldwide suffer from asthma, and this number continues to rise. Asthma represents a significant societal burden, affecting patients' quality of life and necessitating substantial healthcare expenditures [27].Studies have demonstrated a link between type 2 diabetes and lung diseases, including asthma, likely resulting from hyperglycemia, chronic inflammation of varying severity, oxidative stress, and endothelial dysfunction. The frequent co-occurrence of these conditions prompts the search for universal therapeutic strategies, among which GLP-1 receptor agonists represent one potential option [18].
A mouse study conducted by Hur J et al. examined the effects of GLP-1 receptor agonist therapy on eosinophilic bronchitis and airway hyperreactivity in obese mice with asthma. The scientists fed the animals a high-fat diet for eight weeks to induce obesity, and over seven weeks, they sensitized the animals to ovalbumin (OVA). Additionally, the researchers performed provocation tests to trigger airway hyperreactivity and eosinophilic bronchitis. Following these steps, they administered intraperitoneal injections of the GLP-1R agonist liraglutide at doses of 1-2 mg. The treatment lasted four weeks, with the drug given five days a week. The authors evaluated therapy effects on eosinophilic bronchitis activity, the expression of interleukins (IL-4, IL-5, and IL-33), and the activity of the NLRP3 inflammasome, which plays a crucial role in asthma development in obese individuals. Study results showed that liraglutide alleviated eosinophilic bronchitis and airway hyperreactivity by reducing the analyzed interleukins. Additionally, the researchers observed a decrease in NLRP3 inflammasome activity and associated IL-1 expression. Based on these findings, the authors concluded that GLP-1 agonists may exhibit potential anti-asthmatic properties [17].
A retrospective cohort study comparing the frequency of asthma exacerbations in patients with type 2 diabetes and asthma found that the use of different drugs for intensifying antihyperglycemic therapy influenced the incidence of such episodes. The study analyzed treatments involving GLP-1 receptor agonists, SGLT-2 inhibitors (sodium-glucose cotransporter-2), DPP-4 inhibitors (dipeptidyl peptidase-4), sulfonylureas, and insulin. The researchers selected patient groups based on electronic medical records, identifying individuals who initiated treatment with the aforementioned substances. After six months of therapy, they observed that patients receiving GLP-1 receptor agonists experienced fewer asthma exacerbations compared to those using SGLT-2 inhibitors, DPP-4 inhibitors, sulfonylureas, and insulin. Based on these findings, the study's authors concluded that GLP-1 receptor agonists may represent a new therapeutic option for asthma in adults with diabetes [11].
Foer, Dinah et al. conducted a study aimed at determining the effects of GLP-1 receptor agonists on systemic inflammatory markers in the airways of patients with asthma and diabetes. The researchers analyzed serum samples from two patient groups to measure periostin, a biomarker of eosinophilic airway inflammation. The smaller group, consisting of 42 patients, was treated with GLP-1 receptor agonists, while the larger group, including 119 patients, received other forms of therapy. To compare the cohorts, the researchers calculated individual propensity scores for each patient, accounting for key variables, followed by a linear regression analysis adjusted to these values. The analysis found that periostin levels were significantly lower in patients treated with GLP-1 receptor agonists, and that its concentration correlated with total IgE levels. Based on these findings, the researchers concluded that GLP-1 receptor agonists influence type 2 airway inflammation. Additionally, they identified periostin as a potential biomarker for assessing the use of GLP-1 receptor agonists in asthma therapy [9].
A study conducted by Kaplan A and colleagues, based on data from the Optimum Patient Care Research Database (OPCRD), found that the use of GLP-1 receptor agonists in obese asthma patients may provide benefits in disease control. The analysis included a group of adult patients who had been diagnosed with asthma or treated for it within 12 months before receiving a GLP-1 receptor agonist prescription. The researchers did not observe significant differences in the frequency of exacerbations between the studied cohorts. However, they found an improvement in asthma control. Patients receiving GLP-1 therapy were prescribed SABA (short-acting beta agonists) less frequently, had fewer episodes requiring oral corticosteroid therapy, and were hospitalized less often. Additionally, they observed a decrease in asthma biomarkers during GLP-1 treatment. The authors emphasize that these findings should encourage further discussion regarding the use of GLP-1 receptor agonists in asthma treatment [19].
Another study, based on data from the Japanese national database, analyzed the impact of different antihyperglycemic drugs on the frequency of asthma exacerbations in diabetic patients. The analysis included nearly 140,000 patients, assessing both the number of exacerbations and the need for systemic corticosteroids. The researchers found that therapy with GLP-1 receptor agonists correlated with poorer asthma control compared to metformin. However, patients receiving SGLT-1 inhibitors achieved asthma symptom control comparable to metformin treatment [21].
An Aurum A cohort study utilizing data from the UK Clinical Practice Research Datalink (CPRD) found that in patients with diabetes and coexisting asthma, metformin use correlated with approximately a 30% reduction in asthma exacerbation risk. Furthermore, researchers observed that this risk decreased by an additional 40% when a GLP-1 receptor agonist was added to the therapy. The authors suggest that these observed associations may result from mechanisms unrelated to glycemic control, body weight, or asthma phenotype [22].
A meta-analysis including 39 randomized controlled trials (RCTs) aimed to determine the association between GLP-1 receptor agonists and asthma incidence in individuals with type 2 diabetes or obesity. The researchers found that compared to patients not using GLP-1 receptor agonists, those taking these medications experienced a slight reduction in asthma incidence. They conducted sensitivity analyses, considering patient characteristics, study design, drug formulation, duration of action, and drug subtypes, but they did not observe significant correlations. The authors emphasized the need for further research to more precisely determine the relationship between GLP-1 receptor agonists and asthma development risk [37].
The FDA analyzed data from its Adverse Event Reporting System (FAERS) to assess the relationship between GLP-1 agonist therapy and asthma exacerbation or onset risk. The results indicated that some substances in this group were linked to a higher incidence of respiratory-related adverse events, including asthma and similar conditions. The researchers found that semaglutide and liraglutide showed the most negative associations, while exenatide correlated with the highest mortality rate. The study authors emphasized that the observed differences remain unclear and may result from reporting errors, patient characteristics, or specific pharmacological properties of individual drugs. Nevertheless, they recommend that physicians remain aware of the potential risks associated with GLP-1 agonist therapy, and they stress the necessity of further research to ensure patient safety [5].
Table 2. The Impact of GLP-1 Receptor Agonists on Asthma – Analysis of Research Findings and Therapeutic Perspectives
| Authors | Year of Study | Main Findings |
| Hur, J., Kang, J. Y., Kim et al. | 2021 | Liraglutide exhibited anti-asthmatic effects in obese mice, alleviating eosinophilic airway inflammation and bronchial hyperreactivity by reducing interleukin levels and NLRP3 inflammasome activity. |
| Foer, D., Beeler, P. E. et al. | 2021 | Patients using GLP-1R agonists experienced fewer asthma exacerbations compared to those on other antihyperglycemic medications. |
| Dinah Foer,
P Beeler,
Jing Cui et al. |
2020 | GLP-1R agonists influenced type 2 airway inflammation by lowering periostin levels, a biomarker of eosinophilic airway inflammation. |
| Kaplan, A., Heatley, H., Townend | 2025 | In obese asthma patients, GLP-1R agonists improved disease control by reducing SABA use, hospitalizations, and oral corticosteroid treatments. |
| Kimura, Y., Jo, T., Inoue, N et al. | 2024 | GLP-1R agonist therapy was associated with poorer asthma control compared to metformin. |
| Lee, B., Man, K. K. C., Wong et al. | 2025 | Combining metformin with GLP-1R agonists reduced the risk of asthma exacerbations by approximately 40% |
| Zhang, M. Q., Lin, C., Cai, X. L et al. | 2024 | GLP-1R agonist use was linked to a slight decrease in asthma incidence compared to non-users. |
| Cazzola, M., Matera, M. G., Calzetta, L. et al. | 2024 | Some GLP-1R agonists were associated with an increased risk of respiratory-related adverse events, especially semaglutide and liraglutide |
A range of observational and clinical studies suggest that GLP-1 receptor agonists (GLP-1RA), primarily used for treating type 2 diabetes and obesity, may also provide benefits in the therapy of chronic lung diseases such as COPD and asthma.
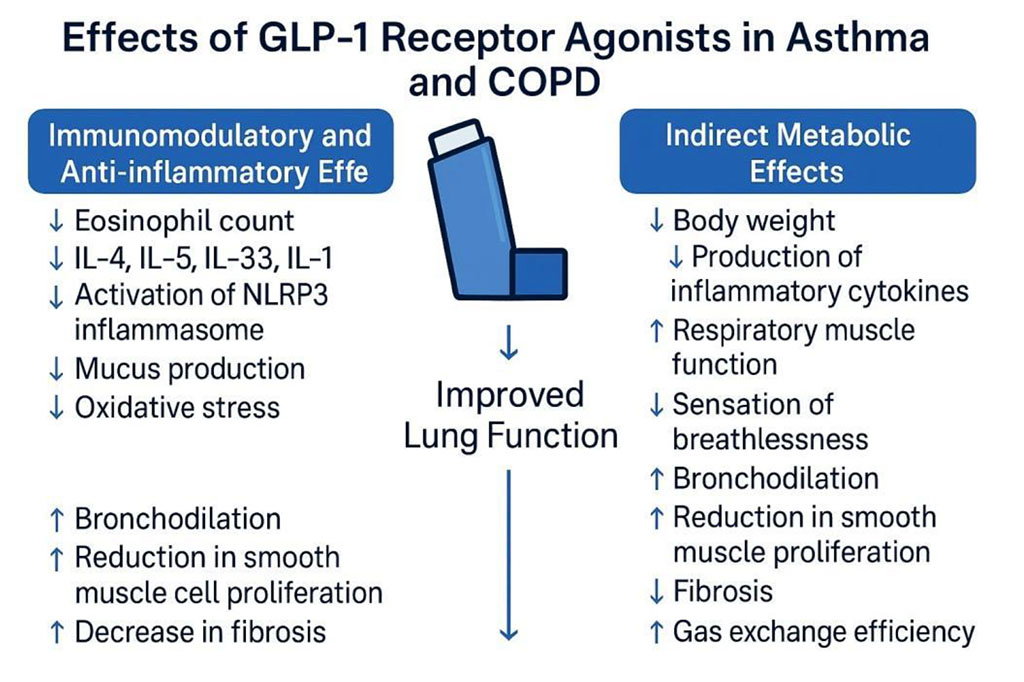
Figure 1. Effects of GLP-1 Receptor Agonists in Asthma and COPD
In the study by Pradhan et al. (2022), GLP-1RA use was linked to a significant reduction in the risk of moderate and severe COPD exacerbations compared to sulfonylurea therapy. The authors suggest that this effect stems from the anti-inflammatory and immunomodulatory properties of these drugs, as well as their impact on weight reduction.
Similar findings were observed in analyses by Albogami et al. and Foer et al., which also confirmed lower hospitalization rates and fewer exacerbations of chronic lower respiratory diseases (CLRD) in patients receiving GLP-1RA.
Additionally, the study by Altintas Dogan et al. (2022) found that liraglutide treatment improved certain pulmonary function parameters (e.g., forced vital capacity (FVC) and diffusion lung capacity for carbon monoxide (DLCO)) and subjective patient symptoms in COPD and obesity, though it did not significantly affect FEV₁ or inflammatory markers.
The latest data by See et al. (2024) further supports the idea that GLP-1 analogs in COPD and T2D patients, even in advanced disease stages undergoing triple inhalation therapy, may reduce exacerbation risk, pneumonia incidence, oxygen dependence, and mortality.
Regarding asthma, researchers have also investigated potential benefits from GLP-1 receptor agonist therapy. In a mouse study conducted by Hur et al., the effects of liraglutide on eosinophilic bronchitis and airway hyperreactivity were analyzed in obese mice with allergen-induced asthma (ovalbumin exposure). GLP-1RA therapy led to reduced airway hyperreactivity, a decrease in eosinophil counts in the lungs, lower expression of interleukins (IL-4, IL-5, IL-33), and suppression of NLRP3 inflammasome activation.
These results suggest that GLP-1 agonists may possess immunomodulatory and anti-inflammatory effects in asthma, opening new therapeutic possibilities, particularly for patients with obesity and chronic inflammatory respiratory diseases.
Based on available research, it can be hypothesized that GLP-1 represents a promising therapeutic approach for the treatment of lung diseases such as asthma and COPD, particularly in individuals with diabetes or obesity [9],[11],[19],[21],[22],[31],[37]. Studies have shown that GLP-1 reduces inflammation in the airways [9][18], mitigates oxidative stress [18], and lowers the levels of specific interleukins [3],[17],[18],[30],[31],[35],[36]. Furthermore, there is evidence suggesting that its use may lead to a decrease in the number of disease exacerbations and related hospitalizations [10],[11],[19],[22],[37]. While current research results are promising regarding disease management, further verification of the safety of long-term use is necessary [5].
Available evidence suggests that GLP-1 receptor agonists may have a positive impact on chronic lung diseases in patients with type 2 diabetes. Although the mechanisms of action are not yet fully understood, they likely involve both anti-inflammatory effects and improvements in respiratory mechanics. However, further research, including randomized clinical trials, is necessary to confirm these findings and establish the role of GLP-1RA in the treatment of respiratory diseases.
All authors contributed equally to this work.
Study conception and design - Aleksandra Sagan, Izabela Domańska; Data collection and/or compilation - Monika Domagała, Weronika Duda, Emilia Majewska, Małgorzata Piśkiewicz, Joanna Wiewióra; Data analysis and interpretation - Monika Domagała, Weronika Duda, Emilia Majewska, Małgorzata Piśkiewicz, Joanna Wiewióra; Manuscript writing - Aleksandra Sagan, Izabela Domańska; Emilia Majewska, Critical review of the manuscript -Aleksandra Sagan, Izabela Domańska, Weronika, Duda, Małgorzata Piśkiewicz, Joanna Wiewióra; Approval of the final version of the manuscript - Aleksandra Sagan, Izabela Domańska, Monika Domagała, Weronika Duda, Małgorzata Piśkiewicz, Emilia Majewska, Joanna Wiewióra