- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 3. DOI 10.35630/2025/15/3.308
Aims: The aim of this study was to investigate whether lung ultrasound (LUS) enables clinicians to differentiate between viral and bacterial community-acquired pneumonia (CAP) in the adult population, and to assess whether the use of LUS can support the decision-making process regarding antibiotic therapy.
Methods: This review was conducted using data retrieved from PubMed and Google Scholar. The search was limited to studies published in English between 2015 and 2025, focusing on the adult population.
Results: LUS demonstrates high predictive value in distinguishing viral from bacterial CAP, reduces diagnostic uncertainty, and supports appropriate initiation or withholding of antibiotic therapy in hospital care. Typical findings for different CAP etiologies have been identified. In both adult and elderly populations, LUS is more sensitive and specific than chest X-ray. In primary care, the combination of LUS and procalcitonin did not significantly reduce antibiotic use.
Conclusions: LUS can effectively assist in the diagnosis of CAP and reduce unnecessary antibiotic prescriptions in hospital care. Typical ultrasound findings, such as the presence of large consolidations, may assist in identifying CAP etiology. However, specific cut-off values for consolidation size that would allow classification of CAP etiology as bacterial have yet to be established. LUS should be preferred over chest X-ray in adults, specifically in geriatric patients. Furthermore, there is a lack of sufficient studies evaluating the use of LUS as a sole diagnostic tool in the primary care setting.
Keywords: ultrasonography, viral pneumonia, bacterial pneumonia, community-acquired pneumonia, geriatrics
Community-acquired pneumonia (CAP) is a common health problem worldwide. The prevalence of CAP varies depending on the source, ranging from 0.32 to 16.9 cases per 1,000 person-years [1,2,3], with incidence particularly high in populations over 85 years of age [3]. While most cases of CAP can be treated on an outpatient basis, 31.8% of patients (especially children and the elderly) require hospitalization [2]. Mortality during hospitalization ranges from 0.7% to 6% [1,2,3]. Furthermore, one study has shown that one-year mortality in hospitalized patients reaches 30.6% [1].
The most common symptoms of CAP usually appear within the first one to two days and include cough, purulent sputum, pleuritic chest pain, dyspnea, chills, fever, and night sweats. However, in elderly patients, symptoms may appear later and be less specific, including confusion, weakness, lethargy, falls, poor oral intake, and decompensation of chronic illnesses. During a physical examination, a physician may find tachycardia, tachypnea, crackles, bronchial breath sounds, and signs of pleural effusion [4].
Regarding the etiology of CAP, most cases are caused by bacterial pathogens, including Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella [4]. However, healthcare practitioners must keep in mind that 18% to 38.6% of pneumonia cases are caused by viral pathogens, with influenza being the most frequently confirmed [5,6]. Establishing the etiology of CAP is crucial, especially in severe cases, as early initiation of antibiotic treatment in bacterial pneumonia reduces in-hospital mortality [5].
In less severe cases, determining CAP etiology is also essential. Lower respiratory tract infections (LRTIs) are a common reason for patient consultations with general practitioners (GPs) [7]. A study by Cheysson et al. (2021) found that 17% of all antibiotics prescribed in outpatient clinics during the cold season were actually used to treat viral LRTIs [8]. Notably, the percentage of antibiotic-resistant pathogens responsible for LRTIs is steadily rising. For example, 30-50% of S. pneumoniae strains in the USA and Spain are multidrug-resistant [9].
Physicians suspecting CAP in a patient can use different imaging methods to confirm the diagnosis. Computed tomography (CT) remains the most accurate diagnostic tool for pneumonia. However, its high radiation dose, limited accessibility in outpatient care, and high cost make it unsuitable for routine use [10]. The most commonly used but less accurate method is chest X-ray (CXR), which has a high specificity but low sensitivity in diagnosing pneumonia [11]. Claessens et al. (2021) found that up to 30% of pneumonia diagnoses based on CXR were not confirmed by CT. Furthermore, a third of patients who had no indications of pneumonia on CXR showed changes on CT [12]. CXR is also associated with significant interobserver variability [1].
Over the past decade, numerous studies have explored the role of lung ultrasonography (LUS) in pneumonia diagnosis. Particularly during the recent SARS-CoV-2 pandemic, LUS was widely used by physicians, not just radiologists [13]. LUS does not expose patients to radiation, can be performed bedside in a short time, and has a high sensitivity and specificity in diagnosing pneumonia [14].
In this review, we aim to investigate whether LUS can help differentiate bacterial and viral lower respiratory tract infections in hospital and outpatient healthcare settings and whether it can contribute to reducing unnecessary antibiotic prescriptions for patients with these infections. The rapid development of ultrasound technology, the growing availability of ultrasound in hospitals and outpatient clinics, and new publications on LUS have motivated us to write this review. While many studies have examined LUS in children, we have found fewer reviews discussing its application in adults. For this reason, we have focused our attention on the adult population.
This review was conducted using data retrieved from PubMed and Google Scholar. The search was limited to studies published in English between 2015 and 2025, focusing on the adult population.
To carry out a lung ultrasound (LUS) examination, physicians can use convex, high-frequency linear, or cardiological probes. The patient is usually asked to remain in a supine or sitting position. Raising the arm above the head helps to widen the intercostal spaces, providing a better view of the lungs [13]. Approximately 20% of the lung surface cannot be visualized using LUS. These include the retroscapular and retrosternal regions, parts of the mediastinal parietal pleura, and the costovertebral recess [13,15]. Because air reflects up to 99% of ultrasound waves, lung examination relies primarily on the interpretation of artifacts [13].
In aerated lungs, the normal ultrasound image consists of hyperechogenic horizontal lines. The line closest to the probe represents the pleura and the subsequent lines appearing at regular intervals deeper in the image are called A-lines. The pleural line moves with respiration—a phenomenon referred to as “lung sliding” [16].
A physician can divide the chest into several zones usually from 5 to 7 for each lung [17, 18]. A numerical score can be assigned to each zone, and the total score across all zones reflects the extent of lung pathology. Common scoring systems include the MLUS, Soldati and modified Soldati scores [17, 19].
Pathological findings in LUS associated with pneumonia include the absence of lung sliding, pleural irregularities (e.g., thickening), B-lines, subpleural consolidations, larger lung consolidations, dynamic air bronchograms, fluid bronchograms, and pleural effusions [20]. B-lines are vertical, hyperechoic lines that arise from the pleura and extend to the bottom of the screen regardless of depth. Multiple B-lines may become confluent. They should be distinguished from Z-lines, which also originate at the pleura but fade a few centimeters into the image. Z-lines can be seen in healthy lungs [21]. B-lines are indicative of decreased lung aeration. The presence of numerous B-lines is commonly referred to as interstitial syndrome [16]. Subpleural consolidations are small (<1 cm wide), hypoechoic, liver-like structures adjacent to the pleura [20]. Larger consolidations (>1 cm wide) are caused by more extensive loss of aeration and can result from infection, infarction, atelectasis, or trauma [16,20]. Air bronchograms appear as hyperechoic linear structures within these consolidations and may be static or dynamic (moving with respiration). The presence of dynamic air bronchograms is highly specific for pneumonia [22,23]. Fluid bronchograms, on the other hand, appear as anechoic tubular structures within consolidations [22]. Pleural effusion is anechoic or hypoechoic.
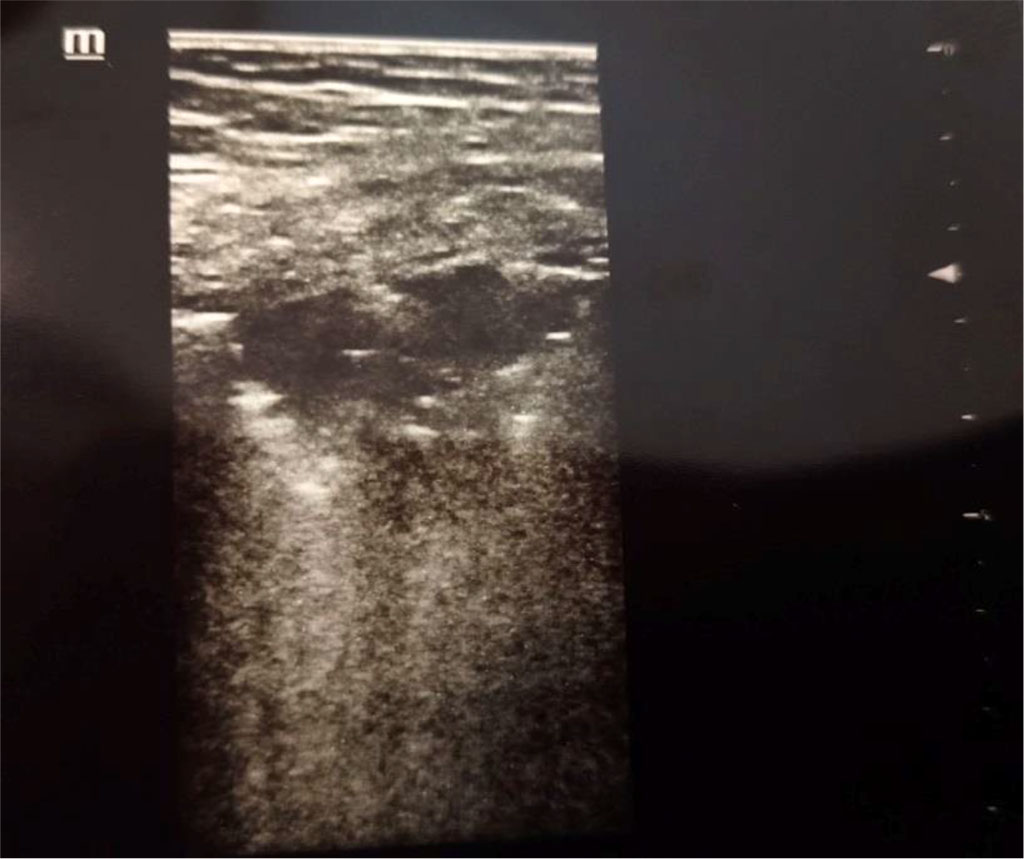
Figure 1. Consolidation. Author’s private materials.
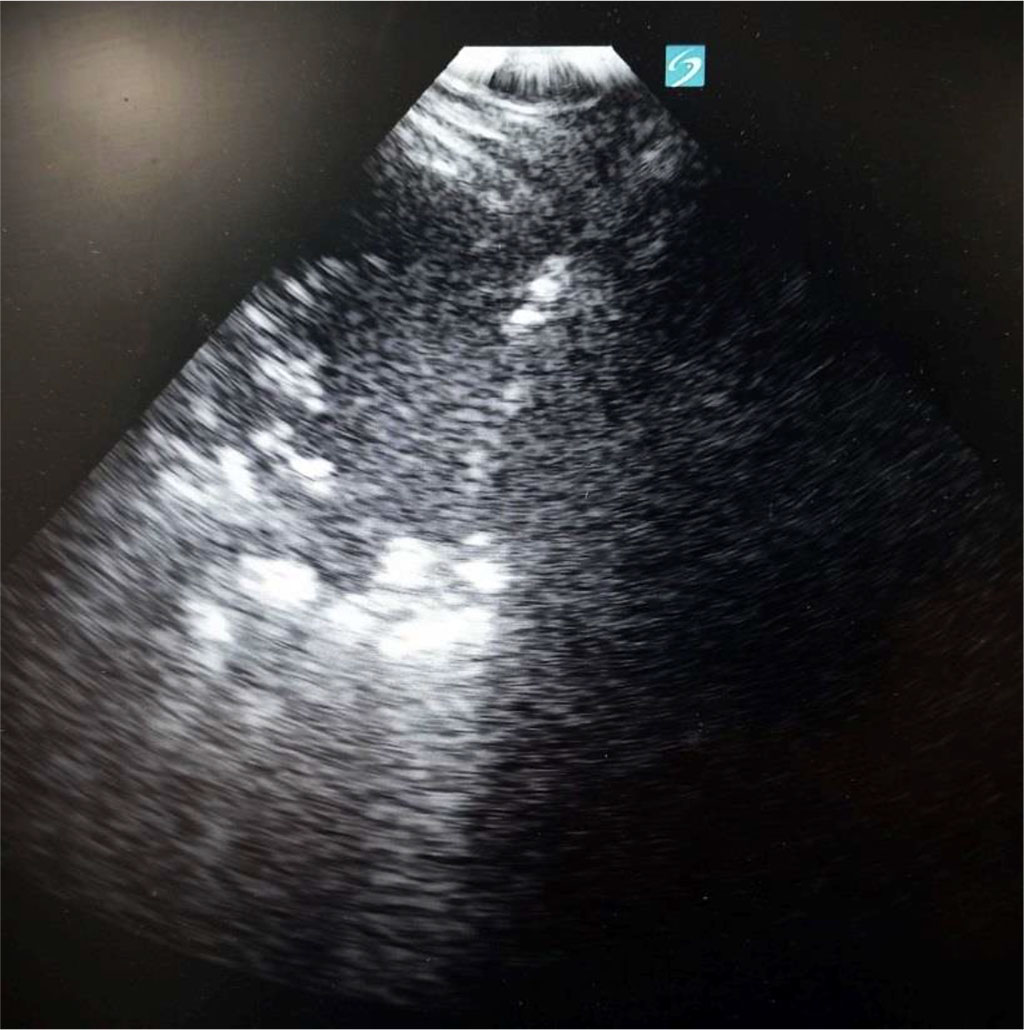
Figure 2. Consolidation with air bronchogram at the left side, diaphragm in the middle and liver at the right side. Author’s private materials.
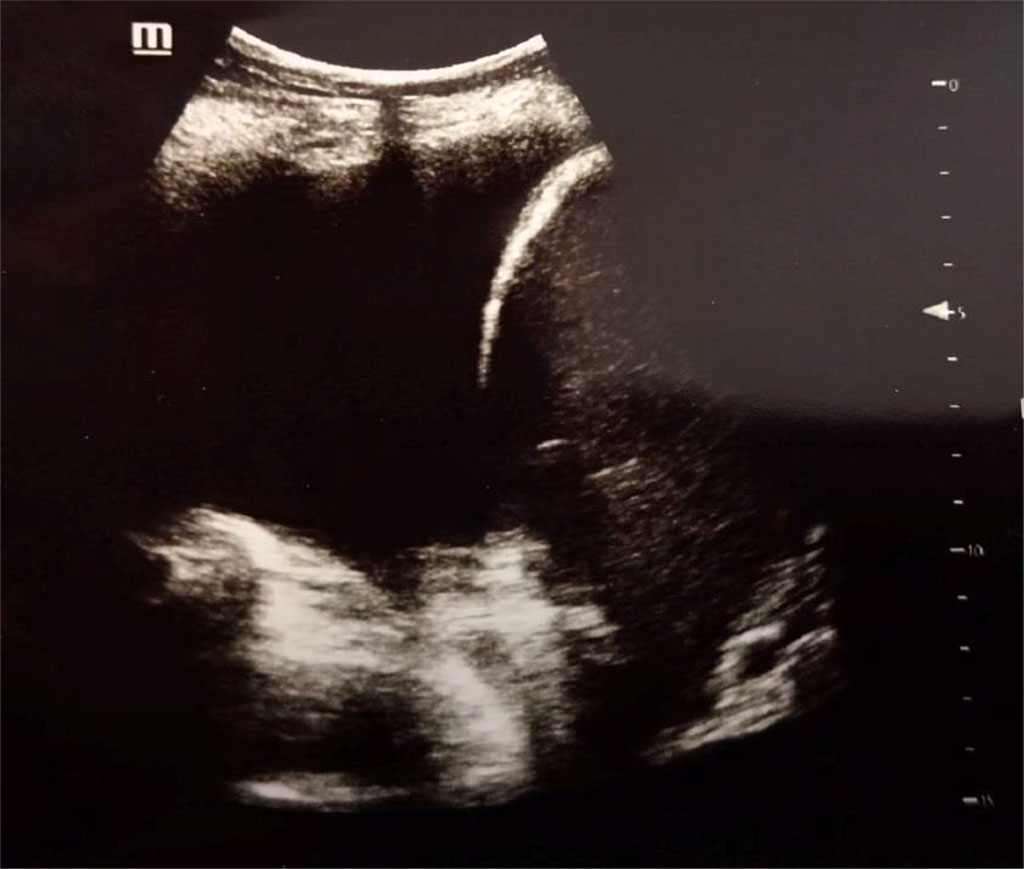
Figure 3. Pleural effusion (anechoic), diaphragm and liver at the right side. Author’s private materials.
A key technical limitation of LUS is that it can only detect abnormalities that reach the pleura. However, in hospitalized adults with pneumonia, approximately 92% of lung changes extend to the pleural surface [24].
Pros of LUS. Meta-analyses have shown that LUS has a high sensitivity (ranging from 0.88 to 0.904) and specificity (ranging from 0.86 to 0.884) for diagnosing pneumonia in adults [25,26]. Comparative studies between LUS and chest X-ray (CXR) reveal that LUS generally offers superior diagnostic accuracy in adult patients with suspected pneumonia [26]. The time required to do LUS examination is estimated to be 3 to 13 minutes, which makes it a perfect tool for rapid assessment both in inpatient and outpatient care [14,27,28]. Additionally, LUS training is rapid - requiring fewer than 10 examinations to perform to achieve a satisfactory efficacy [28].
CAP etiology and LUS presentation. According to New International Guidelines and Consensus on the Use of Lung Ultrasound, LUS is feasible and useful in general/family medicine and in prehospital emergency care. It should be used by clinicians as a point-of-care examination in patients presenting with dyspnea and chest pain [29]. Mearelli et al. (2021) found that LUS, when performed upon admission to an internal medicine ward, is useful in identifying bacterial etiology through the evaluation of consolidations, as well as for ruling out fatal outcomes in patients with CAP [30]. Considering already existing recommendations to extend the use of LUS across a broader patient population, we reviewed studies exploring the utility of LUS not only in the detection of pulmonary abnormalities, but also in establishing the etiology of CAP and in reducing or appropriately adjusting antibiotic prescriptions in patients with suspected CAP.
For instance, Jauvadin et al. (2021) concluded that integrating LUS into routine diagnostic procedures reduced diagnostic uncertainty in CAP cases from 73% to 14%. In their study physicians assigned patients with CAP into a four-level Likert scale (definite, probable, possible and excluded). After adding LUS to routine examination most changes occurred in the probable and possible categories, as the patients were reclassified as “definitive” and “excluded”. Therefore it may be especially useful to consider performing LUS in the inconclusive clinical presentation of CAP. Another important finding is that the implementation of LUS led to changes in antibiotic prescriptions in 32% of patients, where treatment was initiated in 45% and discontinued in 55% of those affected [31].
Mearelli et al. (2021) in the study conducted on 410 patients with definitive diagnosis of CAP, were able to distinguish two patterns based on positive LUS results. Pattern 1 was characterized as one or more subpleural consolidations with or without one or more areas of alveolar-interstitial syndrome. Pattern 2 was described as one or more areas of alveolar-interstitial syndrome. LUS turned out to be a highly reliable tool in predicting the necessity for empirical antibiotic therapy, as the positive predictive value was 99% for bacterial etiology of CAP and 97% for bacterial coinfection in CAP. At the same time, identifying pattern 2 could be correlated to viral etiology and support the decision to withhold antibiotic therapy with a negative predictive value of 93% [30].
In primary care settings, Rodríguez-Contreras et al. (2022) suggested a clinical algorithm for decision-making when using LUS in patients with suspected CAP who do not meet hospitalization criteria. According to their study, consolidation greater than 1 cm should result in antibiotic treatment. Focal B-lines pattern without consolidation greater than 1 cm should indicate the need for an X-ray evaluation and the decision should be made based on symptoms and X-ray results. If A-lines are observed, antibiotic treatment should be withheld unless the patient's condition is worsening [32].
LUS has also shown promise in identifying atypical pneumonia and by that has enabled clinicians to administer more effective antibiotics. As reported by Sayed et al. (2023), LUS may serve as a supplementary diagnostic tool in atypical pneumonia and is particularly useful in situations that require a rapid diagnostic process, such as pandemics of acute respiratory diseases [17].
In the context of tuberculosis, Meli et al. (2023) after analyzing six studies, including 213 patients, were able to identify common ultrasonographic findings in patients with tuberculosis. In the adult patients with tuberculosis, most common findings, present in 90% of adults, were circular or ellipsoidal hypo-echoic subpleural lesions, usually smaller than 1.5 cm, defined as “subpleural nodules”. Lesions were mostly localized in the superior lung quadrants. Common were also consolidations, often numerous and localized in the apical parts of the lung. Another frequent finding was pleural irregularities [33]. Montuori et al. (2019) reported that the combination of apical consolidations and subpleural nodules showed diagnostic accuracy of 0.799 for tuberculosis [34].
The utility of LUS in differentiating between viral infections has also been recently explored. The study conducted by Goldsmith et al. (2022) showed that clinicians may be able to distinguish between influenza and COVID-19 using LUS and the modified Soldati score. COVID-19 diagnosis was more frequently associated with B-lines in all lung zones, irregular pleura and subpleural consolidations. In contrast, pleural effusion was more commonly observed in patients with influenza. The median modified Soldati score was 9 for patients with COVID-19 and 0 for patients with influenza. LUS showed promising results as a method in rapid differential diagnosis of viral infections [35].
Further comparisons were made by Tung-Chen et al. (2022), who analyzed LUS patterns in patients with SARS-CoV-2 pneumonia versus those with CAP of other etiologies. LUS findings in SARS-CoV-2 pneumonia were reported to be very similar to other viral infections of the lower respiratory tract. LUS findings in SARS-CoV-2 pneumonia have been described as subpleural consolidations (smaller size) and more diffuse distribution (higher lung score). Findings more specific to CAP of other etiologies included larger consolidations (hepatinisation) and more localised involvement (lower total lung score). The authors suggest that LUS findings, with a Lung Score greater than 10 and complementary tests, can be effective in predicting the aetiology of pneumonia, particularly in epidemic situations and when rapid diagnostic processes are required [36].
Table 1. Typical ultrasonographic findings in CAP of different etiologies [13,27,34,35,36]. *excluding pneumonia of Mycobacterium tuberculosis etiology, **excluding pneumonia of SARS-CoV-2 etiology
| Study | Disease | Typical ultrasonographic findings |
| Buda et al. (2020) [27] | Bacterial CAP* | larger subpleural consolidations (>25 mm) |
| dynamic or mixed air bronchogram | ||
| normal flow pattern in color Doppler | ||
| pleural effusion (approximate volume > 200 mL) | ||
| Boccatonda et al. (2023) [13] | Viral CAP** | smaller subpleural consolidations (<5mm) |
| diffuse distribution of B-lines | ||
| irregular pleura (thickened > 2 mm) | ||
| small pleural effusion | ||
| Goldsmith et al. (2022) [35], Tung-Chen et al. (2022) [36] | COVID-19 | smaller subpleural consolidations |
| diffuse distribution of B-lines (more present than in other viral CAP) | ||
| irregular pleura | ||
| Montuori et al. (2023) [34] | Tuberculosis | subpleural nodules |
| apical consolidations |
Inflammatory biomarkers and LUS. Biomarkers such as C-reactive protein (CRP) or procalcitonin (PCT) can help with determining the etiology of LRTI [37,38]. These biomarkers are now available in some general practices as well as LUS. In this part of the work we wanted to investigate if there are some correlations between CRP and PCT serum concentrations and LUS findings and show result of two studies comparing the use of PCT and PCT+LUS with usual care (no use of PCT and LUS) in general practitioner work.
In bacterial and mixed (bacterial and viral infection) pneumonia both CRP and PCT values are usually elevated compared to viral pneumonia. PCT has higher specificity (72.9-90% using cut-off value of ≥0.5 μg/L) in differentiating bacterial and viral LRTI than CRP. Prognostic value for mortality in CAP patients is also better using PCT [37,38]. CRP however, has a higher sensitivity in differentiating bacterial infection ( 95.0% with cut-off value of >10mg/L) [37].
Comparing biochemical markers and LUS presentations, higher CRP and PCT values can be found in patients with consolidations (with or without air bronchogram) than in patients with alveolar-interstitial syndrome or patients with no abnormalities in LUS [30].
Unfortunately, we have not found prospective studies comparing the use of PCT/CRP with LUS in the diagnosis of bacterial CAP. However, there are two prospective studies comparing the use of PCT and PCT+LUS with usual care (no use of PCT and LUS) in general practitioner work. Both studies were conducted in collaboration with 60 general practices, using a PCT cut-off value of ≥0.25 μg/L and the presence of consolidation in LUS as indicators for antibiotic treatment. The subjects of both studies were patients with a clinical suspicion of pneumonia [39,40]. In the Lhopitallier et al. (2021) study, PCT-guided antibiotic therapy decreased the number of prescribed antibiotics and chest X-rays. No further reduction in antibiotic prescriptions was noted in the PCT+LUS group [40]. In the Cisco et al. (2024) study, the use of PCT-guided antibiotic therapy was shown to decrease antibiotic prescription rates with no additional expenditures compared to the "usual care group" (no PCT and no LUS use). The use of PCT+LUS showed no advantage over PCT alone in reducing antibiotic prescription rates. Additionally, the PCT+LUS group was characterized by significantly higher costs [39].
Although the number of general practices using LUS is growing, the cited studies show that the use of PCT alone is sufficient to decrease antibiotic prescriptions in patients with suspected pneumonia, with the same effect as using PCT+LUS examination. There is a lack of prospective studies comparing the usual management (without inflammatory biomarkers and LUS use) with LUS examination regarding antibiotic prescriptions.
Diagnosing and treating CAP in geriatric population may be especially challenging, as elderly patients often do not present with classic pneumonia symptoms [32] and the diagnostic value of common biomarkers and prognostic tools is different than in the younger population [41]. At the same time, both the incidence rate and the mortality rate of CAP increase with patients' age [42]. The article written by Markarian et al. (2019) showed that implementing early LUS in patients over 64 years old with dyspnea can allow clinicians to effectively predict clinical severity, represented by the need for intensive care unit admission and/or death within 48 hours after admission to the emergency department [19]. Another study, performed by Buda et al. (2020), compared sensitivity and specificity of LUS and CXR in elderly patients (defined as older than 65 years) hospitalized due to suspected pneumonia. LUS was found to be more effective at revealing pulmonary inflammatory lesions in this population [27]. Other studies conducted on geriatric patients are also presenting LUS as superior diagnostic tool compared to CXR, particularly in terms of sensitivity in revealing pulmonary consolidation caused by CAP [43,44]. Scarlata et al. (2023) emphasize that LUS may be a key diagnostic tool in acute cases among the elderly, as it is less affected by patient non-cooperation due to cognitive impairment, which is more prevalent in geriatric population, compared to CXR. However, they also highlight concerns regarding the lack of universally accepted standards for equipment, procedures, and reporting, despite the promising results of numerous studies [45].
Lung ultrasound (LUS) demonstrates superior diagnostic performance compared to chest X-ray in adult patients with suspected community-acquired pneumonia (CAP) [26], including the elderly population [27,43,44]. Moreover, LUS is easy to learn, requires 3 to 13 minutes to make, and is less dependent on patient cooperation—making it especially valuable in bedridden patients and geriatric care [14,27,28].
The use of LUS in clinical practice may reduce diagnostic uncertainty and assist in the decision to initiate antibiotic therapy in inpatient care [30,31]. The presence of consolidations, especially with dynamic air bronchograms, suggests a bacterial etiology of CAP [30,32]. Identifying this pathology via LUS may guide the decision to start empirical antibiotic therapy more effectively than relying solely on chest X-ray findings. However, studies report varying cut-off sizes for consolidation considered specific for bacterial CAP, with thresholds ranging from 10 mm to 25 mm [27,32]. Viral infections are more likely in patients with B-lines, small subpleural consolidations (<5 mm), irregular pleura, and small pleural effusions [13,30,34]. Depending on the clinical presentation, these patients may not require antibiotic therapy, or further diagnostic evaluation may be warranted. To differentiate COVID-19 pneumonia from other viral infections, the Soldati score can be applied, with higher scores being more suggestive of COVID-19 [35].
Despite the clear correlation between elevated CRP and PCT levels and LUS-detected consolidations [30], the combination of PCT and LUS has not shown added benefit in reducing antibiotic prescriptions in primary care compared to PCT alone [39,40]. Furthermore, the LUS + PCT strategy has been shown to be less cost-efficient [39,40]. Notably, data comparing the effectiveness of PCT and LUS in primary care remains limited.
Lung ultrasound (LUS) is an effective and accessible diagnostic tool for the evaluation of community-acquired pneumonia (CAP), particularly in hospitalized adults and geriatric patients. Compared to chest X-ray, LUS offers superior diagnostic accuracy, faster bedside assessment, and greater sensitivity for detecting pulmonary consolidations. LUS findings—especially the presence of large consolidations (>1 cm), dynamic air bronchograms, and pleural effusion—may support the diagnosis of bacterial CAP and guide timely initiation of antibiotic therapy. In contrast, diffuse B-lines and small subpleural consolidations are more suggestive of viral pneumonia and may support decisions to withhold antibiotics.
Further research should aim to validate LUS-based algorithms in various clinical environments, define quantitative thresholds for CAP etiology differentiation, and assess long-term clinical outcomes associated with LUS-guided antibiotic therapy.