- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 3. DOI 10.35630/2025/15/3.305
Aim: To present a clinical case where laser photobiomodulation (PBM) was used as an adjunct to soft tissue augmentation with autologous connective tissue grafts.
Case Description: A 42-year-old non-smoking female patient suffering from type II diabetes presented with thin gingival phenotype in the upper anterior region. A subepithelial connective tissue graft (SCTG) was harvested from the palate and placed using a tunneling technique. Adjunctive PBM therapy was applied using a diode laser (λ=635 nm, 100 mW, 4 J/cm²) at multiple time points.
Clinical Results: Postoperative healing was uneventful, with reduced discomfort (VAS) reported. At 1, 2-weeks and 3-months follow-up, significant gains in keratinized tissue width and gingival thickness were observed.
Conclusion: The postoperative application of PBM represents an innovative and effective approach that supports tissue regeneration and enhances patient well-being. Research findings indicate that this therapy has a positive impact on the body's biological mechanisms, which is particularly important for individuals with impaired healing processes.
Keywords: diode laser, photobiomodulation, connective tissue graft, thin gingival biotype, gingival thickness, width of keratinized gingiva
Implant procedures in the aesthetic zone of the maxilla represent a major challenge for dentists and often require additional soft tissue augmentation procedures. The gingival biotype, the width and height of the soft and hard tissues in the area to be implanted, as well as the condition of the periodontal tissues and the teeth adjacent to the edentulous area play an important role. These factors are of great importance not only for aesthetic reasons, but also influence the degree of alveolar bone loss around the implant. To improve aesthetic conditions and thickness of the gingival phenotype, subepithelial connective tissue grafts (sCTG) taken from the patient's hard palate are often used [1,2]. Autogenous sCTG grafts are widely used in mucogingival plastic surgery and have become a reliable method used to: increase keratinised tissue width (KTW) [3], treat recessions [4], treat furcations [5], manage implant soft tissue [6], or reconstruct gingival papillae [7]. In order to thicken the gingival phenotype, it is possible to take sCTG from the patient's palate, but this involves the creation of two treatment fields, which contributes to greater postoperative discomfort. Despite this, sCTG is still considered the ,,gold standard" in mucogingival plastic surgery [8-12].
Photobiomodulation (PBM) is the therapeutic use of non-ionising radiation, absorbed by endogenous chromophores, triggering biological reactions, based on photophysical and photochemical (not thermal) processes, leading to physiological changes in cells. PBM is a non-invasive, painless technique with low risk to the patient and no side effects [13]. In order to achieve a deep tissue effect, the properties of electromagnetic waves in the therapeutic window range, corresponding to wavelengths from 600 to 1100 nm, are applied [14,15]. A significant factor influencing the extent of energy absorption within the tissue is the type of treatment technique employed. The most favourable outcomes are achieved through the utilisation of the contact method, with the laser applicator positioned perpendicularly to the surface of the irradiated tissue. In the context of open wounds, it is imperative to employ a non-contact technique for the purpose of irradiation. PBM can be used as a standalone treatment (monotherapy) or as an adjunctive therapy to conventional dental treatment (pharmacotherapy or physiotherapy). The synergistic combination of PBM with non-steroidal anti-inflammatory drugs has been shown to be of particular benefit [16-18]. PBM leads to analgesic, biostimulatory and anti-inflammatory effects in tissues. The analgesic effects of PBM have been demonstrated to reduce the patient's pain. This phenomenon can be attributed to the hyperpolarisation of neuronal membranes, alterations in the concentration of neurotransmitters at synapses, and an augmentation in the secretion of β-endorphins. It has been demonstrated that the capacity of PBM impulse conduction is associated with a reduction in central sensitisation, which in turn results in a sustained reduction in pain [14-16,19-22]. The biostimulatory effect is the result of changes resulting from the application of PBM improving circulation, nutrition and cell regeneration. Furthermore, it has been demonstrated to stimulate protein synthesis, fibroblast growth and collagen production (which is crucial in the process of wound healing), and also affects the stimulation of angiogenesis [14-16, 25]. The mechanism of the anti-inflammatory action of PBMs mainly involves stimulation of increased cytokine production, which causes vasodilation and stabilisation of osmotic pressure, as well as inhibition of the increase in vascular permeability. These mechanisms lead, in the acute phase of inflammation, to the inhibition of oedema build-up as well as the formation of granulation tissue - newly formed connective tissue rich in capillary networks, while in the chronic phase of inflammation, they enable the formation of a peripheral circulation, drainage of inflammatory foci, absorption of exudate and disappearance of oedema [13-15,25].
The aim of this article is to present a clinical case study of the use of a 635 nm diode laser for photobiomodulation (using a non-contact technique) of the treatment field, performed during the postoperative treatment phase, to accelerate tissue healing, reduce pain and improve adaptation of a connective tissue graft used for peri-implant soft tissue augmentation in the aesthetic zone of the upper jaw.
The patient, aged 42 was reffered from another dental office for peri-implant soft tissue augmentation in the aesthetic zone of the maxilla in the area of the implant located in position 13. Her history included type 2 diabetes, pharmacologically stabilised (she was taking Metformin 750mg twice a day), and no known allergies. Family history, no genetic burden. On extraoral clinical examination, no apparent facial asymmetry, chin and submandibular group A lymph nodes not palpable, not painful. Trigeminal nerve outlets not painful. No pathology in the temporomandibular joint. Intraoral clinical examination revealed a thin gingival biotype and a insufficient transverse gingival dimension in the vicinity of the implant at the crown neck of tooth 13, with no signs of inflammation, bleeding, bacterial plaque or pathological depths at the 6 points examined around the implant (Fig.1). The thickness of the keratinised gingiva at the implant at position 13 was measured by ultrasonography. The thickness of the keratinised gingiva was 0.75 mm (Tab.1). The width of the keratinized gingiva in the specified area was 1 mm (Tab.1).
The patient was qualified for peri-implant soft tissue augmentation using the tunnel technique and a subepithelial connective tissue graft (sCTG) taken from the palate. The procedure was discussed, post-operative recommendations were explained and written informed consent was obtained for the procedure. One Shot - 2g of Amoxycyllin was administered 1h before surgery - perioperative antibiotic prophylaxis. Prior to the procedure, the patient had blood drawn from the ulnar vein to obtain Advanced Platelet Rich Fibrin (A-PRF) membranes to secure the donor site located on the palate (Fig.2). Infiltration anaesthesia of the donor site and conduction block anaesthesia of the greater palatal orifice with articaine 1:100000 was administered. With use of preoperatively prepared template (Fig.3), an epithelial-connective tissue graft was harvested from the palate using the free gingival graft (FGG) technique (Fig.4). A larger amount of sCTG was harvested due to the need to simultaneously cover gingival recessions in the left maxillary region. The graft was then de-epithelialised (Fig.5). The donor site wound was secured with an A-PRF membrane and crossed 5-0 non-resorbable mattress sutures (Fig.6). Under infiltration anaesthesia of the recipient site with a 1:100,000 preparation of articaine in area 12-14, a supraperiosteal tunnel was dissected (Fig.7). An sCTG was then inserted into the tunnel (Fig.8) and 6-0 non-resorbable sutures were placed to position and stabilise the graft in the intracoronal position (Fig.9). Oral rinsing with 0.1% chlorhexidine digluconate for 2 weeks, and postoperative instructions were prescribed. Wound toileting was performed at the follow-up visits each time.
The patient was was qualified for PBM therapy with the 635 nm Smartm Pro diode laser (Lasotronix, Piaseczno, Poland) due to the presence of delayed wound healing in diabetic patients, and the benefits of this therapeutic modality. The nature of the procedure was explained, the stages of treatment were discussed and written consent was obtained for the procedure. The laser settings used were: laser output power 100 mW, fluence 4 J/cm2, CW-continuous wave mode, power density (0.199 W/cm2), single irradiation time is 20 seconds (Fig. 10) and angled glass applicator, flat surface, 8 mm diameter (surface area 0.5024 cm2), non-contact, point-by-point method (14 application points in total), point spacing 2 mm. (donor site 7 points (Fig.11) and recipient site 5 points (Fig.12)). The total duration of a single irradiation session was 240 seconds and the total energy was 48 J. PBM was applied immediately after the surgical procedure and on days 3, 7, 10 and 14 after surgery (Tab.2), for anti-oedematous effects, accelerated tissue healing and improved patient comfort.
After 7 days, the sutures were removed from the donor site region. The patient reported complaints of pain in the area of the donor site (in the area of the recipient site, these were almost imperceptible). A non-violent swelling was observed on the right side of the anterior maxilla. After two weeks, the sutures from the recipient site were removed and normal healing of the palate and recipient site was observed (patient continued to complain of donor site tenderness) (Fig.13). The condition of the soft tissues after a period of 3 months was aesthetically satisfactory, both for the operator and the patient, and the thickness of the keratinised gingiva over the implant in position 13 measured was 1.5 mm (Tab. 1). The width of the keratinised gingiva in the indicated area increased by 2.5 mm (Tab.1). A procedure was performed to expose the implant with augmentation of the surrounding soft tissues using the roll-flap technique, and after a period of one week, a temporary crown was placed (Fig.14).
Table 1. Changes in Clinical Parameters Over the Follow-Up Period
| Time | Gingival Thickness (mm) | Width of Keratinized Gingiva (mm) | Discomfort (VAS) |
| Before Operation | 0.75 | 1 | - |
| 1 Weeks After | 1.8 | 3 | 5 |
| 2 Weeks After | 1.7 | 2.5 | 3 |
| 3 Months After | 1.5 | 2.5 | 0 |
Table 2. Laser Parameters and Structure of Postoperative Care
| Session | Day Post-op | Wavelength (nm) | Power (mW) | Energy Density (J/cm²) | Application Mode |
| 1 | 0 | 635 | 100 | 4 | Contact |
| 2 | 3 | 635 | 100 | 4 | Contact |
| 3 | 7 | 635 | 100 | 4 | Contact |
| 4 | 14 | 635 | 100 | 4 | Contact |
In the case described here, the surgical procedure was supplemented with the use of a 635-nm diode laser for photobiomodulation using a non-contact technique. This resulted in accelerated healing of the recipient and donor sites, with no inflammatory complications. There was a rapid improvement in patient comfort and a satisfactory aesthetic result in the opinion of the patient and the doctor. The measurements revealed an augmentation in the thickness of the keratinized gingiva over the implant by 0.75 mm (Tab.1) and an increase in the width of the keratinized gingiva by 1.5 mm (Tab.1). The present case is consistent with previous findings by Dipalma et al. suggesting that PBM can reduce postoperative discomfort and accelerate soft tissue healing [23]. Prabhu et al. confirmed the effect of PBM on increased fibroblast mitosis, collagen synthesis, epithelialization and granulation tissue formation, thus contributing to accelerated healing [24]. In order to achieve the full regional effect, the surrounding lymph nodes and lymphatic ducts should also be irradiated, thus improving lymphatic drainage comprehensively [25]. Another advantage of photobiomodulation is that it can be used when pharmacotherapy is contraindicated due to allergies. Following surgical procedures in the oral cavity, alternative techniques to PBM may be employed to promote healing and tissue regeneration. Such techniques include LED light therapy, ozone therapy, the use of bioactive dressings (e.g., A-PRF), or topical probiotic therapy. [26,27].
A physician willing to use this method is obligated to have knowledge of the anatomy, proper cycle planning and an individual approach to each patient. The most important factors are appropriate dosimetry taking into account the phase of inflammation, the age and condition of the patient, an adequate therapeutic interval and the application of the most suitable tissue irradiation technique.
The limitations of this case study are twofold. Firstly, a histological evaluation was not performed, and secondly, no control group was included for comparison with other cases. While the clinical results are encouraging, further research in the form of randomised, controlled preclinical and clinical studies is required to establish optimal treatment protocols. It is imperative to note that there exists an absolute contraindication to PBM in areas where malignancy is suspected or confirmed. The limitations of this treatment modality and the indications for considering alternative therapies include: the limited depth of light penetration, the presence of necrosis/major inflammation (PBM works best on living cells), excessive wound area, the presence of dressings/physical barriers through which light does not penetrate effectively, and insufficient patient cooperation.
PBM in a postoperative context offers a contemporary and efficacious approach to facilitating the healing process and enhancing patient comfort. PBM has been demonstrated to exhibit beneficial biological effects, a property that is of particular importance in patients suffering from healing disorders. It is evident that PBM does not constitute a replacement for the patient's conventional postoperative management protocol, but is a valuable addition to it.
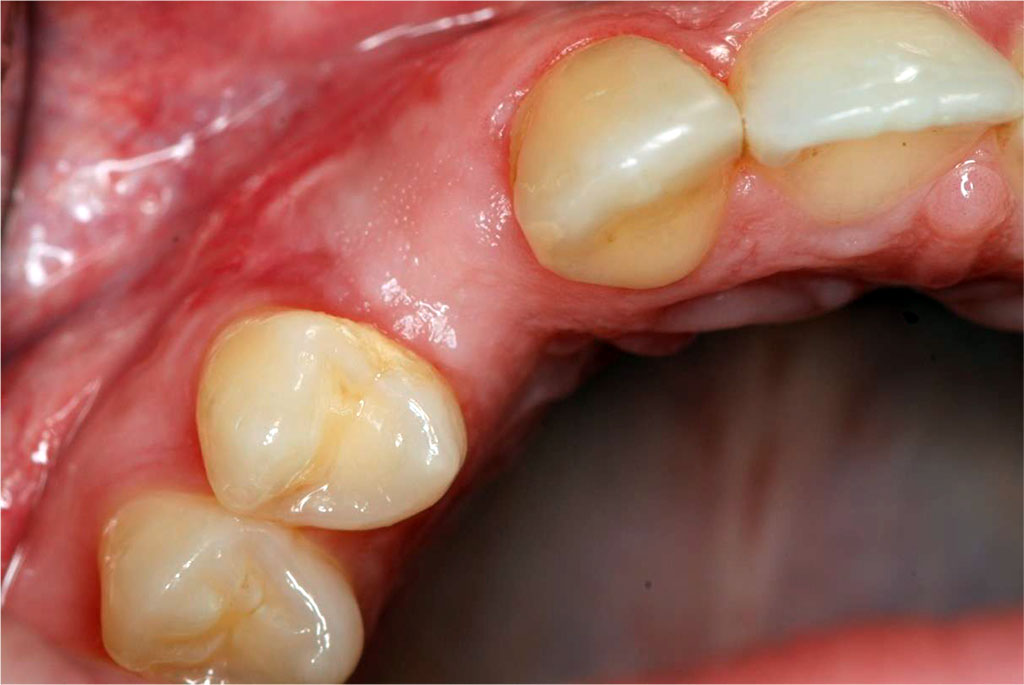
Fig.1 The condition of the peri-implant soft tissues before sCTG augmentation.
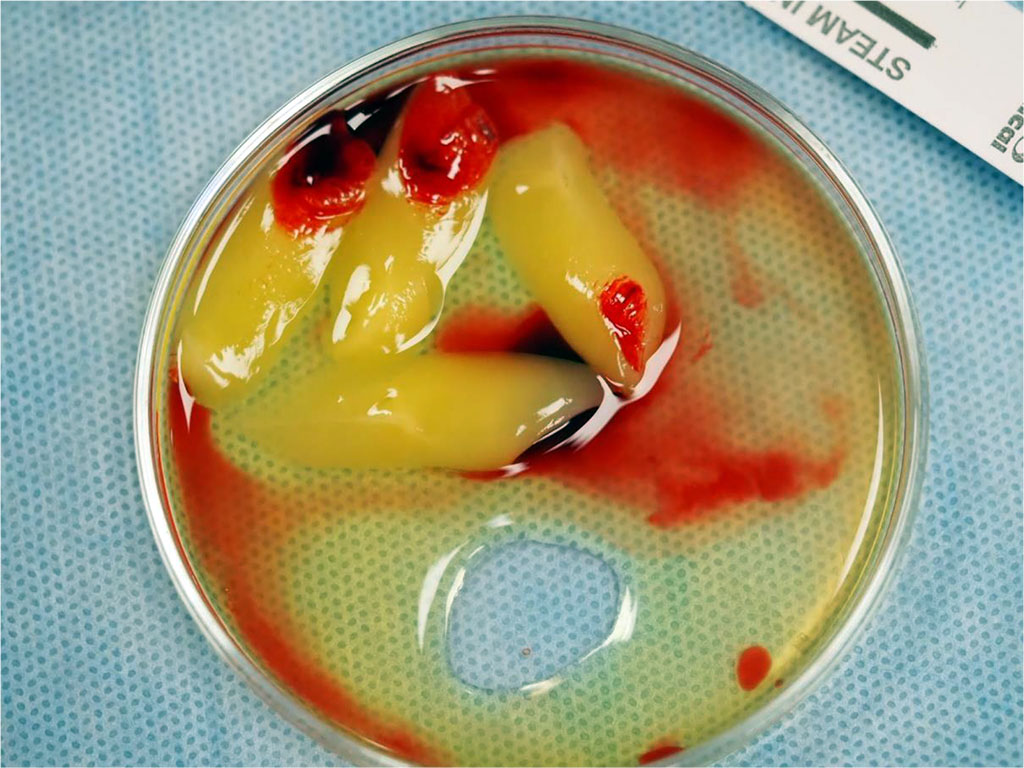
Fig.2 Before the procedure, blood was drawn from the patient's antecubital vein and then centrifuged to obtain advanced platelet-rich fibrin (A-PRF) membranes, which were used to protect the donor site on the palate.
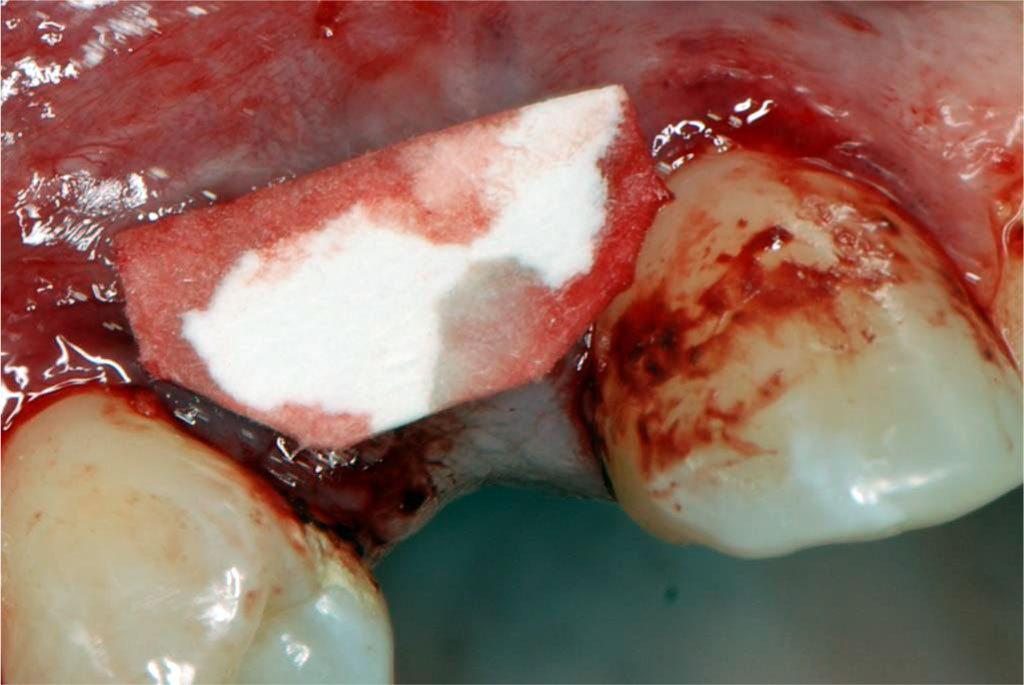
Fig.3 The template size was determined based on the recipient site to accurately harvest the required amount of tissue from the donor site.
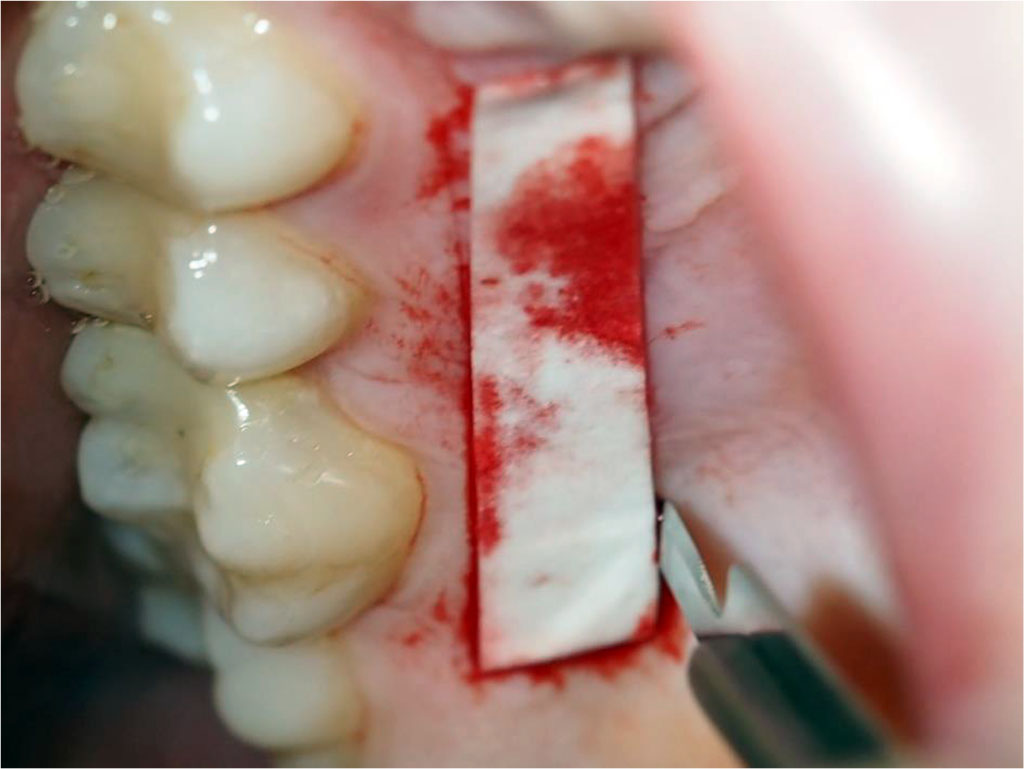
Fig.4 Harvesting an sCTG graft from the palate using the free gingival graft technique.

Fig.5 The epithelial-connective tissue graft was de-epithelialized after harvesting, resulting in an sCTG.
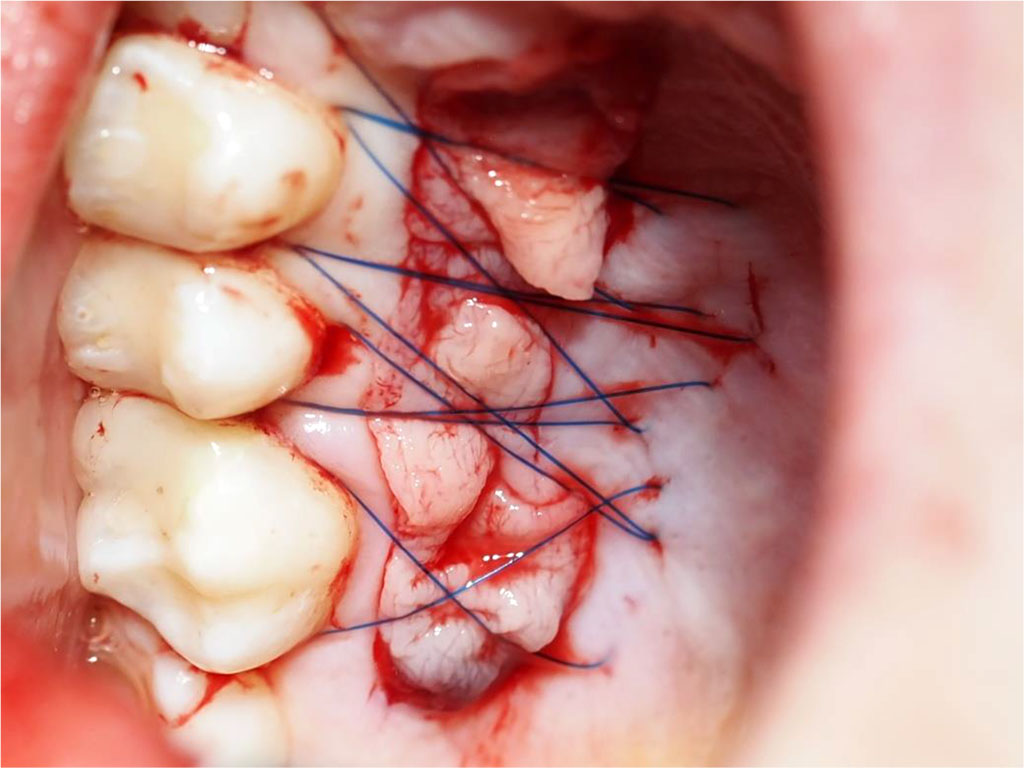
Fig.6 Post-suturing condition of the palatal wound (secured with crossed mattress sutures using non-resorbable 5-0 thread).
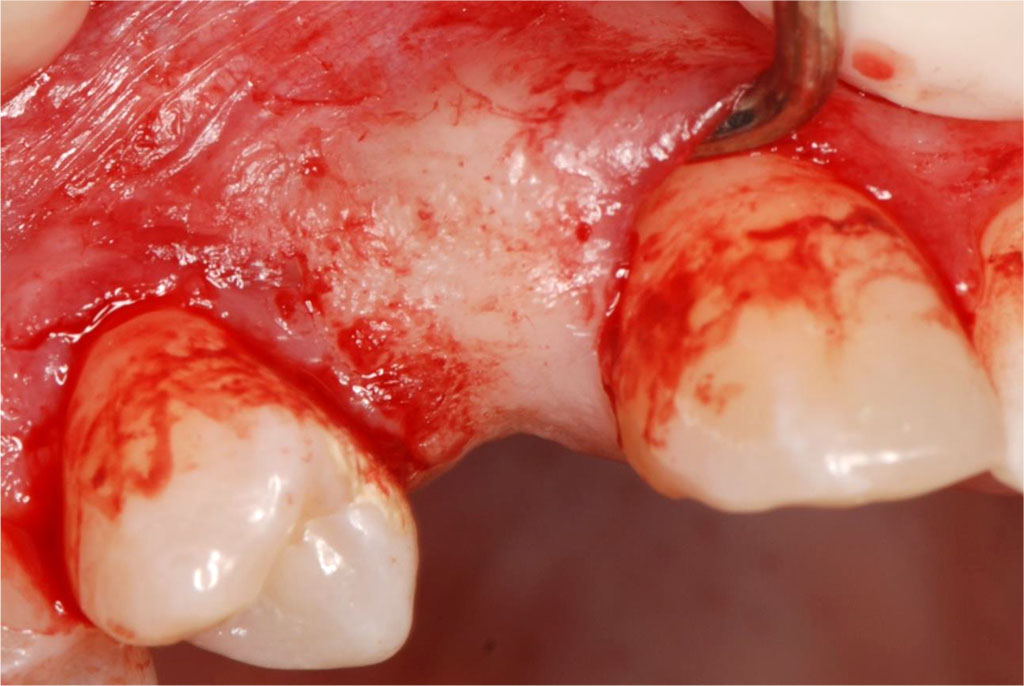
Fig.7 Tunnel preparation in the area of 12-14 using a tunneling instrument set. (Helmut Zepf Medizintechnik GmbH, Seitingen-Oberflacht, Germany).
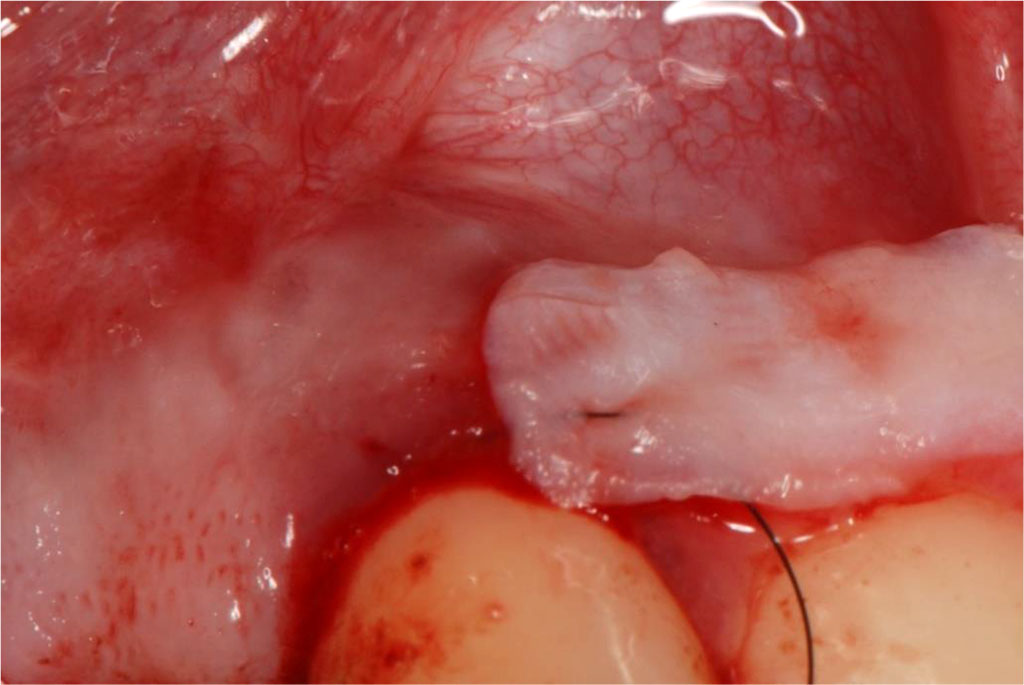
Fig.8 Insertion of the sCTG into the prepared tunnel.
_html_3d842e0a.jpg)
Fig.9 Condition after placing sutures at the recipient site.
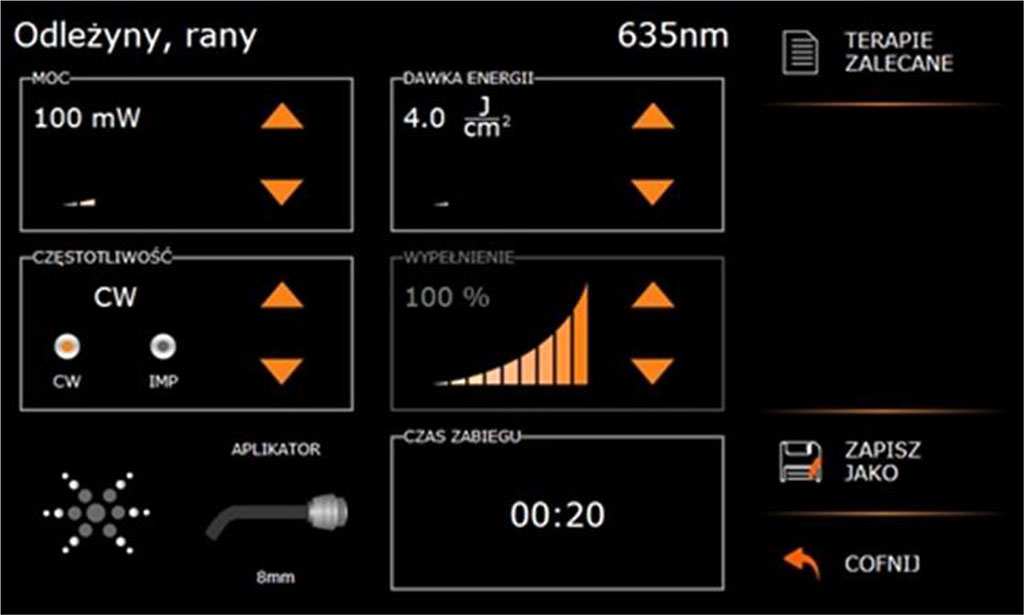
Fig.10 Laser settings used for the PBM procedure (with a glass applicator surface area of 0.5024 cm², the fluence is 4 J/cm²)
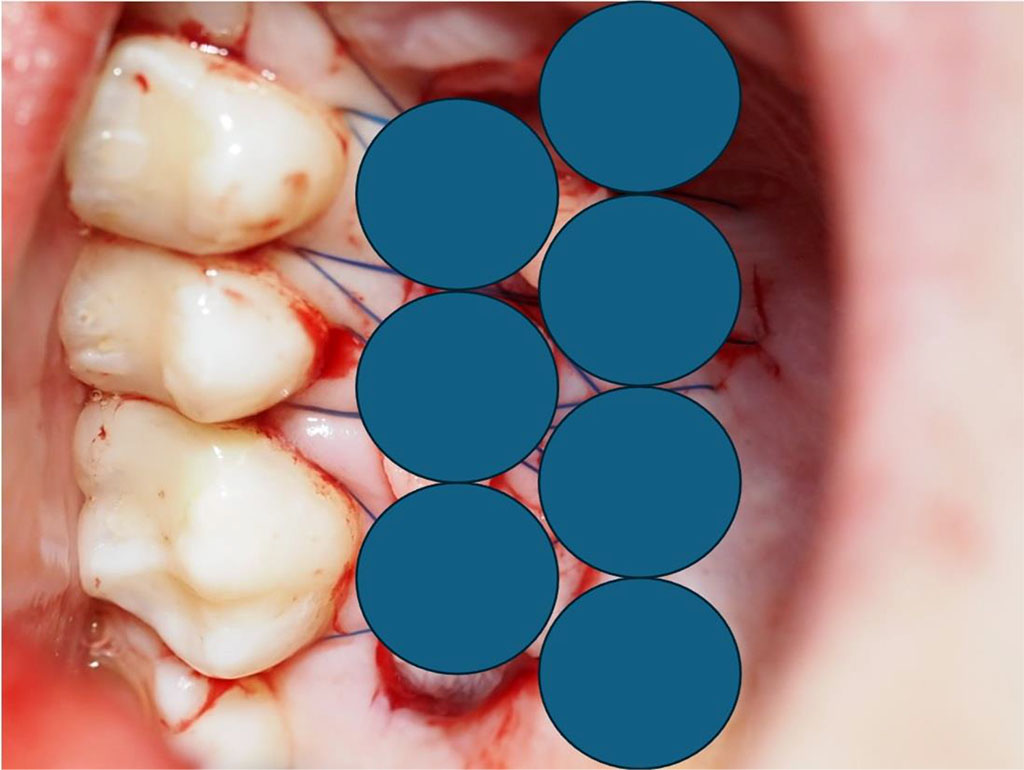
Fig.11 Diagram showing 7 intraoral points of non-contact irradiation of the donor site.
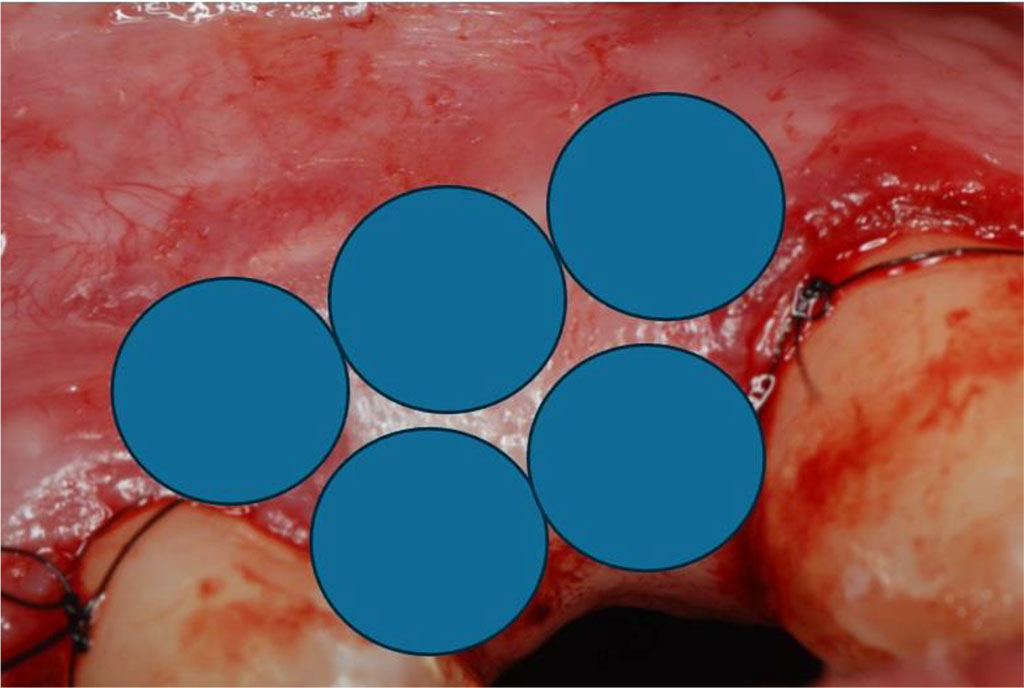
Fig.12 Diagram showing 5 intraoral points of non-contact irradiation of the recipient site.
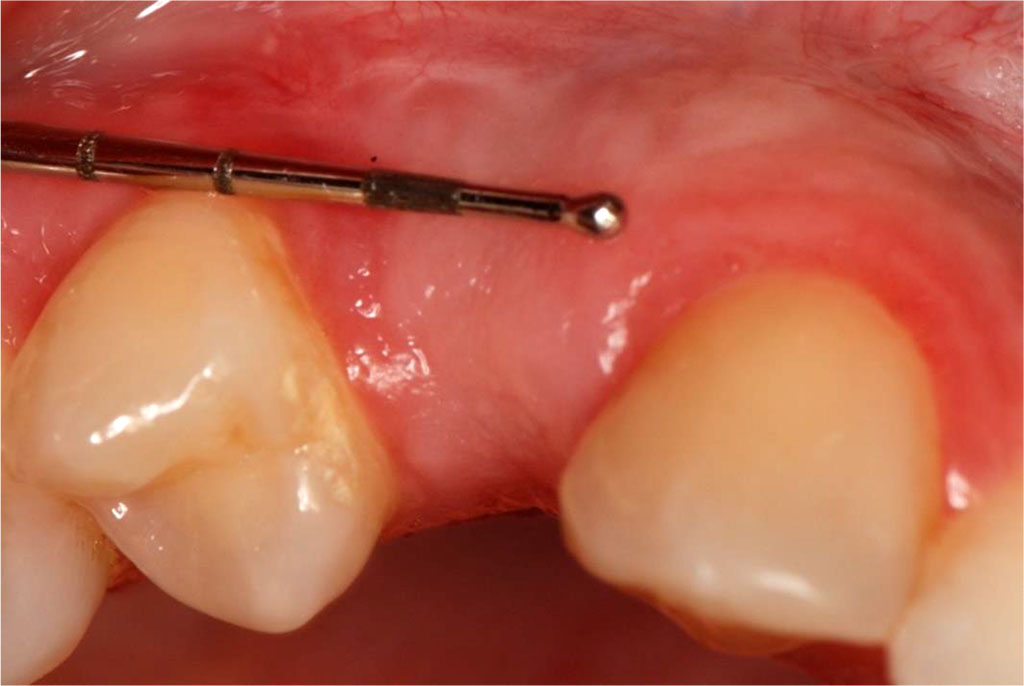
Fig.13 Tissue condition one month after the surgical procedure.
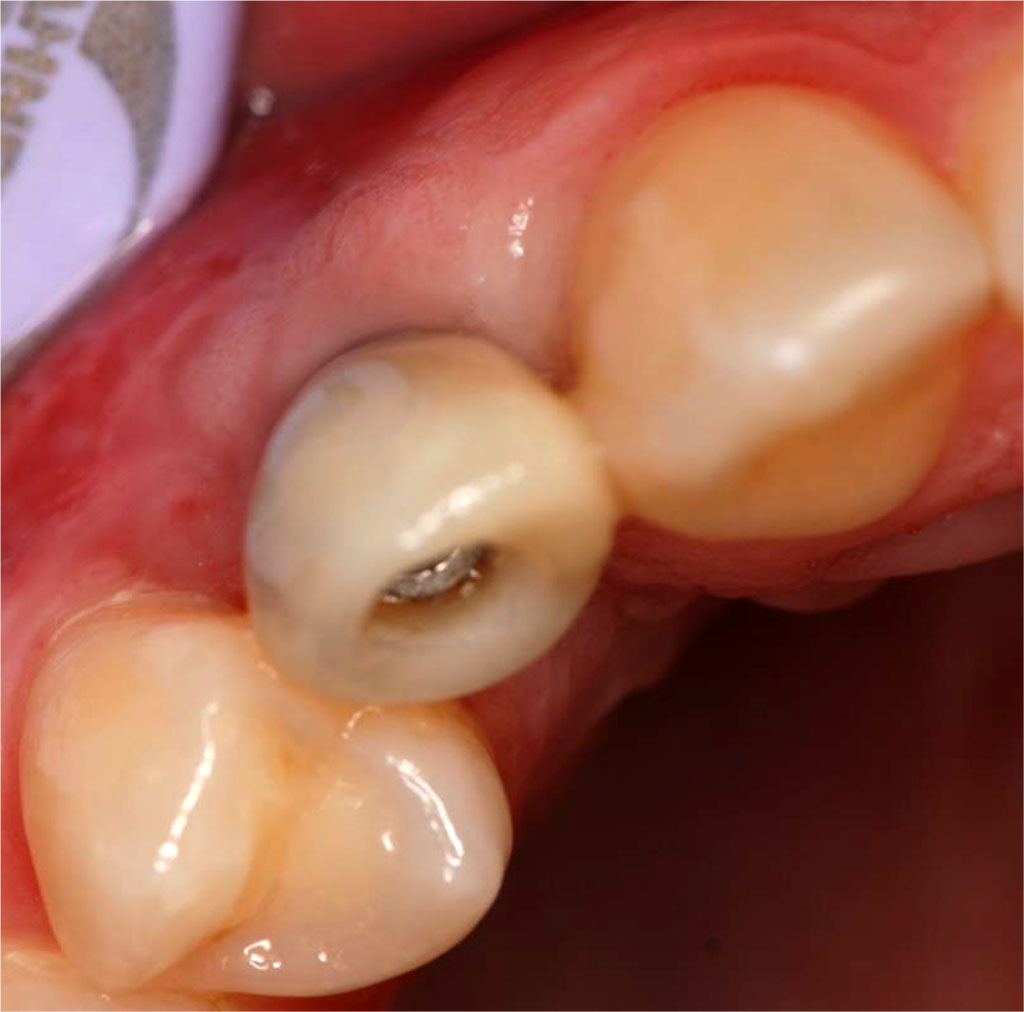
Fig.14 Tissue condition after augmentation procedures with a temporary crown in place (occlusal view).