- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 2: e1. DOI 10.35630/2024/14/2.209
The article presents a clinical case report and a review of the literature regarding massive pulmonary embolism and perioperative cardiac arrest during nephrectomy for malignancy, with massive thrombus in the inferior vena cava. The case report discusses a patient undergoing nephrectomy due to malignancy who experienced sudden cardiac arrest during surgery caused by massive pulmonary embolism. The authors of the article present a description of the surgical and rescue procedure performed. The literature review includes an analysis of similar cases and management strategies for massive pulmonary embolism and perioperative cardiac arrest. The authors evaluate various methods of therapeutic interventions. The work is an important source of information for medical practitioners, especially surgeons and anesthesiologists, who may encounter similar clinical situations. The presented case and literature review may be used to better understand and manage similar medical emergencies.
Keywords: Pulmonary embolism, nephrectomy, anesthesiology, cardiac arrest
Intraoperative acute pulmonary embolism (PE) is a rare but potentially fatal surgical complication that requires urgent medical intervention. A case study of PE during nephrectomy provides an important source of scientific interest, allowing us to explore the mechanisms and risk factors associated with this complication in the context of a specific surgical procedure.
In this article, we present a case study of a patient who experienced intraoperative acute pulmonary embolism during nephrectomy. The analysis of this case will allow us to thoroughly assess the circumstances that may have contributed to the occurrence of PE, including possible predisposing factors and the surgical procedures used.
During nephrectomy, especially in major surgeries, there are a number of factors that may increase the risk of PE. These include, among others: long-term immobilization of the patient, manipulation of the veins, as well as possible vascular injuries. Case study analysis will allow us to identify these factors and assess their impact on the development of pulmonary embolism.
Analysis of this case study will be a valuable source of information for both surgeons, anesthesiologists and medical staff involved in the care of patients undergoing nephrectomy, enabling a better understanding of the risks associated with this procedure and improving preventive and therapeutic strategies aimed at minimizing the occurrence of PE.
An 83-year-old male patient was admitted to the Department of Urology due to a tumor in the right kidney with a tumor plug in the inferior vena cava (VCI) for treatment. A kidney tumor was detected during cardiac diagnostics due to decreased exercise tolerance. The patient was diagnosed with heart failure with an EF of 45-50%, tricuspid valve regurgitation, moderate aortic and mitral valve regurgitation, permanent atrial fibrillation, and arterial hypertension. He also underwent treatment for bilateral kidney stones approximately 30 years before the current hospitalization and adenomectomy 25 years ago. During hospitalization, a lesion in the kidney was confirmed by computed tomography - the right kidney was rebuilt nodularly, with cysts, without infiltration of the surroundings, a plug/thrombus in the renal vein penetrating into the VCS (?!). Left kidney with cortical cysts up to 18 mm, SCI not dilated, N0M0 within the scope of examination. Additionally, an ultrasound examination was performed with the following images: the right kidney was enlarged, remodeled, with cysts, the left kidney was normoechoic with cortical cysts up to 2 cm, the bladder was empty. After performing the necessary laboratory tests, the patient was qualified for surgery. The patient was under general anesthesia, lying on his back with his right side raised. An incision under the left and right costal arches reaches to the sternum. The kidney with the tumor, the ureter and the vascular pedicle were dissected. Many additional vessels were clamped and ligated. Tumor infiltrates along the entire length of the inferior vena cava. Intraoperative ultrasound was performed - a clot extending to the liver, above the liver without a plug. The (previously embolized) renal artery was cut off. The renal vein was dissected along its entire length to the inferior vena cava. Tapes were placed on both sides of the renal vein, under the plug and on the hepatoduodenal ligament. All veins (below, above and laterally) were closed with a turnicle. The renal vein was tied off and the right kidney was cut off. The cut of the inferior vena cava was widened. Infiltration of the decaying tumor into the wall (cancer infiltration into the vein wall was cut off sharply). The material was removed by additional flushing above the lesion. The inferior vena cava was sutured to remove air before final closure. The venous cuffs were released completely, and no blood leakage was found at the place where the vein was sewn. Complete hemostasis was performed. During the layered suturing of the wound, the patient's vital functions broke down. Resuscitation was initiated in accordance with current ERC guidelines. An arterial blood gas (ABG) test was performed and repeated at half-hourly intervals throughout the resuscitation process. Detailed results, including resuscitation time, are presented in Table 1.
| SCA | 30 minutes | 1 hour | 1.5 hours | 2 hours | |
| FiO2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| pH | 7.047 | 7.1 | 6.9 | 6.79 | 6.65 |
| pCO2 | 73.7 | 79.4 | 83.1 | 97.6 | 97.8 |
| past 2 | 33.4 | 36.1 | 39.6 | 41.8 | 40.5 |
| HCO2 | 19.8 | 17.1 | 15.5 | 14.5 | 14.3 |
| B.E.(B) | -11.1 | -16.2 | -18.7 | -20.3 | -20.5 |
| ctCO2 | 22.1 | 22.0 | 19.8 | 17.5 | 20.1 |
| CO OXIMETRY | |||||
| Hct | 30 | 30 | 28 | 29 | 25 |
| tHb | 10.3 | 10.0 | 10.2 | 10 | 10.2 |
| SO2 | 41.2 | 42.2 | 43.5 | 44.2 | 43.1 |
| FO2Hb | 41.0 | 41.5 | 42 | 43.7 | 43.5 |
| FCO2Hb | 0.3 | 0.5 | 0.5 | 0.8 | 0.9 |
| FHHb | 58.4 | 57.9 | 56.1 | 55.2 | 55.2 |
| nBili | 1.8 | 1.7 | 1.5 | 1.3 | 1.3 |
| ELECTROLYTES | |||||
| Na+ | 136.3 | 136.2 | 138.1 | 140.1 | 142 |
| K+ | 6.23 | 6.53 | 6.91 | 8.7 | 9.2 |
| Ca++ | 1.16 | 1.16 | 1.12 | 1.03 | 0.97 |
| Cl- | 107 | 105 | 105 | 104 | 102 |
| METABOLITES | |||||
| Glu | 330 | 315 | 320 | 312 | 340 |
| Lact | ---?↑ | 9.0 | 9.4 | 9.6 | 12 |
Based on the clinical picture, pulmonary embolism was suspected. An urgent cardiology consultation was called. Echocardiography in the transthoracic projection confirmed the features of massive overload of the right ventricle and the image of an empty left ventricle.
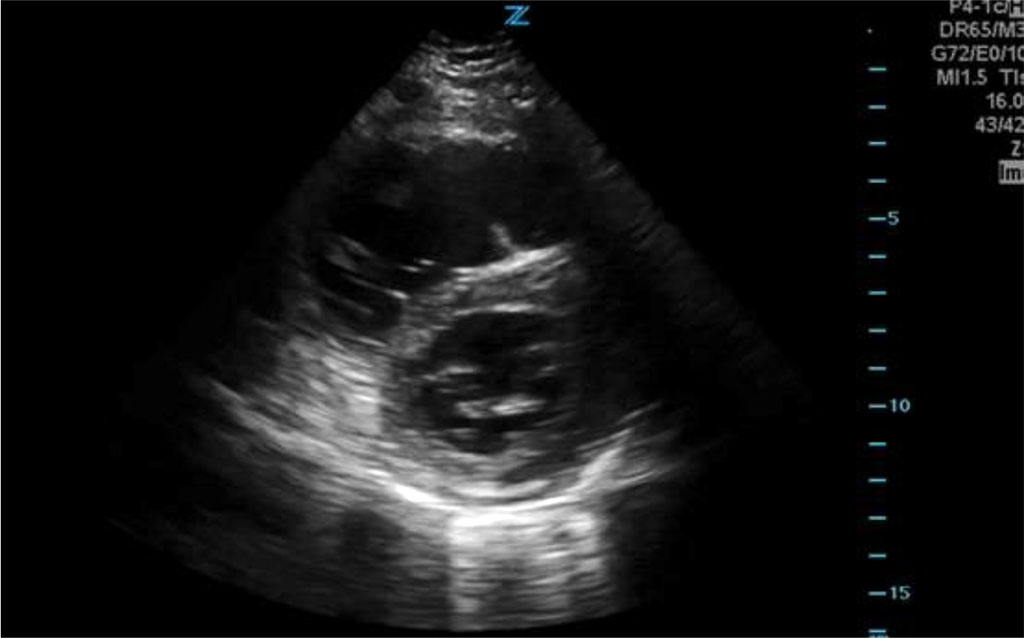
Fig.1 Echocardiographic image showing right ventricular overload
Treatment was implemented in accordance with the recommendations of the interventional cardiologist - Actilyse. 80 mg. The patient was resuscitated for a total of 120 minutes using the LUCAS 3 system. Due to the lack of return of spontaneous heart rate and profound hemodynamic failure, the patient was pronounced dead.
Surgery puts patients at increased risk of pulmonary embolism (PE). Anesthesiologists may be responsible for diagnosing and treating this sometimes fatal disorder. Moreover, the diagnosis is often based on exclusion and may be obscured intraoperatively by much more common disorders, including bleeding and infection. Ongoing surgery may limit initial management options. Pulmonary embolism poses various challenges in surgical patients. During surgery, PE often initially presents with hemodynamic instability and is more likely to progress rapidly, leading to death within hours. However, prompt diagnosis and treatment can reduce morbidity and mortality[1,2,3].
The incidence of PE during and after surgery increases as much as fivefold. In addition to risk factors unique to the perioperative period, patients undergoing surgery have multiple patient-specific risk factors for PE, including acute inflammatory response due to tissue injury, activation of the coagulation cascade, and venous immobilization/stasis. In the study by Goldhaber et al., 50% of patients who developed perioperative PE received thromboembolic prophylaxis. Pulmonary embolism occurs in approximately 0.3–1.6% of the general surgical population. In 2009, Beyer et al. reported that the incidence of PE after prostate surgery was 5.8%. The incidence of PE is estimated at 0.7–30% after all orthopedic surgery and 4.3–24% after hip fracture treatment, mainly due to the location of the surgery, which may distort the femoral vein, leading to impaired venous return and stagnation[4,5,6].
Most studies do not distinguish between intraoperative and postoperative emboli, but Koessler et al reviewed 4 series of patients undergoing total hip arthroplasty and found that the incidence of symptomatic intraoperative PE ranged from 0.6% to 10%. The mortality rate associated with perioperative PE is as high as 12.9% in patients presenting for treatment of a hip fracture. However, both Kerkez et al. and Milbrink and Bergqvist recently found a much lower incidence of symptomatic emboli, approximately 0.25%, in patients receiving thromboembolic prophylaxis after hip fracture surgery[7,8]. In contrast to the high rate of perioperative PE in orthopedic patients, laparoscopic procedures are associated with a low incidence of both nonfatal and fatal PE [9,10,11].
This reduction is hypothesized to occur as a result of less surgical trauma, previous ambulation, and a less pronounced prothrombotic state in laparoscopic procedures than in open surgical procedures. Some of the change in the incidence of postoperative PE over the past 10 years can be attributed to advances in the detection of smaller and more peripheral emboli. The incidence of postoperative PE increased from 2.3 to 9.3 per 1000 in unselected cancer patients between 2000 and 2005. This increase correlated with an increase in the use of spiral (spiral) computed tomography (CT) from 6.6 scans per 1,000 postoperative patients in 2000 to 45 scans per 1,000 postoperative patients in 2005 and was almost exclusively attributed to improved detection. Increased detection was limited to segmental and subsegmental emboli. About 10.7% of patients diagnosed in 2005 had no symptoms. The incidence of fatal PE in this series remained stable at 0.4 per 1000 patients each year, suggesting that there was no change in the incidence of severe emboli [12,13].
Abnormalities in the functioning of the respiratory system and gas exchange in the lungs are some of the first changes observed when venous emboli are placed in the lungs. These are attributed to an increase in alveolar dead space or, more directly, to right-to-left shunting and V/Q mismatch, which may be a consequence of physical obstruction to blood flow caused by the emboli themselves. When blood flow is diverted away from obstructed pulmonary arteries (PAs), it causes overperfusion of the remaining lung tissue, leading to edema, loss of surfactant, and alveolar hemorrhage. The resulting atelectasis develops acutely but may persist long after the emboli have resolved and the lungs have been reperfused. Hypoxia due to pulmonary lesions may be much more severe in the presence of a foramen ovale patent to the probe, opened by increased pressure in the right atrium (RA) and causing a large interatrial shunt. In patients with low cardiac output (CO), low mixed venous pO2 may exacerbate the impact of V/Q shunt or mismatch on arterial oxygenation. However, increasing CO with inotropic drugs can also reduce pO2 by increasing shunt blood flow. Regional hypocarbia can lead to bronchoconstriction, as can humoral mediators such as serotonin released from platelet-rich emboli [14,15].
The initial hemodynamic disturbance associated with PE is obstruction of blood flow caused by emboli in the pulmonary vessels and pulmonary outflow tracts, leading to an acute increase in right ventricular (RV) impedance. Right ventricular outflow impedance may also be increased by pre-existing pulmonary disease, as well as by neural reflexes and the release of pulmonary vasoconstrictors into the bloodstream. Pulmonary vasoconstrictors, such as serotonin and platelet-activating factor, come from platelets trapped in the emboli themselves. Vasoactive peptides, such as activated complement factors 3 and 5, are derived from plasma, and histamine is derived from tissue mast cells. Acute increases in pulmonary vascular resistance and RV afterload may begin a cycle of deleterious changes in the circulation.
Due to its geometry, the RV is much more sensitive to pressure loads than to volume loads. Therefore, an increase in pressure load will lead to a significant reduction in right ventricular (SV) stroke volume. To maintain CO, the initial body response is catecholamine-induced tachycardia and an increase in RA pressure and RV preload to restore normal SV. However, increasing RV preload often leads primarily to RV dilatation and subsequent leftward shift of the interventricular septum, limiting left ventricular (LV) filling. As afterload and RV pressure continue to increase, especially in the face of RV ischemia, the RV begins to fail and CO begins to decline. As the RV pumps less blood through the narrowed pulmonary vessels, LV preload will decrease, reducing LV output to the systemic circulation. Initially, catecholamine-induced vasoconstriction will increase systemic vascular resistance and maintain systemic arterial pressure (BP). However, further reduction in CO concentration leads to systemic hypotension [16,17].
Over 100 years ago, Virchow described his now famous triad of risk factors for venous thromboembolism (VTE): venous stasis, endothelial damage, and a hypercoagulable state. All PE risk factors can be linked in some way to this central concept. Patients with cancer deserve special attention. They are associated with an increased risk of malignancy due to the induction of a hypercoagulable state caused by both hormones released from the tumor and certain chemotherapy agents. Cancer patients are also affected by limited mobility, the frequent presence of central venous catheters, and possible venous obstruction caused by the tumor. Cancer is such a strong risk factor for VTE that a recent meta-analysis by Carrier et al showed that patients with cancer had a rate of 30 VTE events per 100 patient-years compared with 12.8 events per 100 patient-years in the general population, and the rate of PE cancer patients with a central venous catheter was estimated at 15–25%[18].
A prospective study by Goldhaber et al. showed that obesity (BMI N 29 kg/m2) and smoking (N 35 cigarettes/day) are independent risk factors for PE in women, with a relative risk of PE of 2.9 and 3.3, respectively. . Some medications can also induce a hypercoagulable state in patients. Specifically, the risk of PE in women using second-generation oral contraceptives is three times greater than in nonusers, and hormone replacement therapy is associated with approximately twice the risk of PE. A recent study by Lacut et al. showed a 3.5-fold increase in the incidence of VTE in patients taking antipsychotic drugs[19].
The choice of anesthesia technique can have a huge impact on a patient's risk of VTE. A meta-analysis by Rodgers et al. comparison of epidural anesthesia (as primary anesthetic or adjunct to general anesthesia) with general anesthesia alone showed a reduction in the incidence of deep vein thrombosis (DVT) and PE of 44% and 55%, respectively, in the group of patients receiving epidural analgesia. The second meta-analysis examined the effect of continuous lumbar epidural analgesia on thromboembolic complications during hip surgery, knee surgery, prostatectomy, and lower extremity vascular surgery, finding a 34% reduction in events in the epidural analgesia group. However, when the effect of thoracic epidural analgesia was assessed for major abdominal and thoracic surgery in the same meta-analysis, no significant difference was observed in the epidural analgesia group. This difference in epidural effect is hypothesized to be due to the fact that thoracic epidural analgesia has a less significant effect on lower extremity blood flow and venous stasis than lumbar epidural analgesia [20].
Although symptoms commonly seen in conscious patients are often helpful in the initial diagnosis of PE, they are masked in anesthetized and mechanically ventilated patients. The anesthesiologist must then rely on other results that may still occur in unconscious patients. Hypotension and tachycardia are classic intraoperative symptoms associated with PE. Significant hemodynamic instability was found in 59% of hospitalized patients with severe PE - 18% presented with cardiac arrest and 10% with hypotension requiring vasopressor therapy. Significant physical symptoms that may be detected by the anesthesiologist include: sharp and low-volume arterial pulse, tachycardia (HR N 100 beats per minute), increased pressure in the jugular veins, gallop rhythm on the left sternal border and an enhanced second heart sound. Wheezing is often mentioned as a physical symptom that may occur in acute PE. However, wheezing occurs to the same extent in patients with confirmed PE as in those in whom PE has been ruled out. The presence of shock is an early and reliable predictor of mortality in patients with acute PE, regardless of whether shock is caused primarily by massive PE or smaller PE in hospitalized patients with pre-existing severe cardiopulmonary disease. Kuchera et al. found a nearly fourfold increase in 90-day all-cause mortality in patients with shock compared with normotensive patients. The “shock index” (HR/systolic blood pressure) N 1 had a significant positive association with in-hospital mortality and was helpful in classifying patients with acute PE into high- and low-risk groups. Furthermore, patients in shock have a significantly greater risk of death within the first hour; diagnosis and initiation of treatment must occur much faster than in hemodynamically stable patients [21].
At best, PE is a diagnosis of exclusion. During and after anesthesia and surgery, diagnosis may be even more difficult. However, common intraoperative monitors and diagnostic tests perhaps slightly modified and combined with a high index of suspicion, can lead to at least a presumptive diagnosis, even during surgery. This early diagnosis is invaluable in planning management and reducing morbidity. Most patients with PE show some abnormalities on electrocardiography (ECG). Geibel et al. 83% of patients with a confirmed diagnosis of PE had sinus tachycardia and atrial arrhythmias; especially atrial arrhythmias were associated with higher mortality. Other common ECG changes associated with PE include ST segment and T wave abnormalities. Nonspecific ST changes, ST segment elevation and depression, or T wave inversion are found in approximately 50% of patients. Complete right bundle branch block and T wave inversion in the precordial leads are the observations that best correlate with the severity of PE [23].
Typical arterial blood gas (ABG) changes found in spontaneously breathing patients with PE include hypoxemia, respiratory alkalosis, and hypocapnia. Systemic arterial hypoxemia is the most sensitive sign of PE and perhaps the only abnormality on ABG in patients with obstruction of 25% or less. In patients without previous cardiopulmonary disease, 74% of patients had PO2 values in room air less than 80 mmHg, and the severity of PE was inversely linear with arterial PO2. In patients whose room oxygen saturation was less than 95% at the time of diagnosis, the incidence of complications was higher[23].
Cardiomegaly is the most common finding on chest radiograph in patients with PE. Although a chest radiograph is not usually very helpful in establishing the diagnosis of PE, it can confirm or rule out alternative causes of hypoxemia and hypotension, such as pneumothorax, pleural effusion, pneumonia, atelectasis, aspiration, or heart failure, that were found on the chest radiograph. in more than 50% of patients with symptoms related to PE [24,25].
If the patient is not currently undergoing surgery and is hemodynamically stable, several options are available to confirm or exclude PE. Traditionally, V/Q scanning is considered the first-line test. However, the vast majority of V/Q scans in the 1990 Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study were non-diagnostic, with only 15% being "normal" and 13% "high probability", leaving 72% as " indefinite". More recently, single-photon emission computed tomography (SPECT) imaging has improved the predictive value of V/Q scans, with only 1% of cases now being nondiagnostic. Angiography has long been considered the "gold standard" in the diagnosis of PE. It is expensive, invasive and not uniformly available. It's not without risk either. Stein et al. demonstrated a 4% incidence of major complications (including death and renal failure) in a population of intensive care unit patients undergoing angiography. Spiral or helical computed tomography continues to gain popularity because it is non-invasive, comparably inexpensive, and readily available. The overall sensitivity of detecting PE using spiral CT is approximately 85%[26].
Pooled analysis of several large series performed showed that spiral CT is significantly more sensitive (94%) and specific (94%) for detecting PE in central arteries. In cases of PE associated with hemodynamic instability or features of RV overload, the sensitivity and specificity of spiral CT reach 100%. A pooled analysis of over 2,500 patients by Schoepf et al showed a greater than 99% negative predictive value associated with normal helical computed tomography. Spiral computed tomography significantly increases the probability of diagnosing segmental and subsegmental PE in postoperative cancer patients. As mentioned earlier, the study in this series was so sensitive that 10.7% of patients diagnosed with peripheral PE on spiral computed tomography were asymptomatic. Echocardiography is not the preferred test to confirm the presence of PE. Embolism was visible on transesophageal echocardiography (TEE) in only 26% of patients with severe PE [27].
However, TEE may be particularly useful to the anesthesiologist because it is readily available and can be performed in the operating room without interrupting the surgical procedure. The speed and accuracy of TEE make it an attractive choice, as demonstrated by Pruszczyk et al. These researchers showed that with an average examination time of 9.6 minutes, the sensitivity of TEE was 80.5% and the specificity was 97.2% in a population of patients with clinical suspicion of PE and confirmed features of the RV strain. TEE compares favorably with spiral CT in establishing the diagnosis of acute central PE. Rosenberger et al. found that TEE detected right ventricular dysfunction in 96%, tricuspid regurgitation in 50%, and left atrial septal deviation in 98% of patients with PE that was severe enough to require surgery. When assessing PE on TEE, the most common finding is RV dilatation. However, at least 30% pulmonary vascular occlusion is required to induce RV dilatation. Additionally, RV dilatation may result from other disease states such as cor pulmonale with pulmonary hypertension, right ventricular infarction, or chronic/recurrent PE[28].
RV dilatation associated with cor pulmonale or chronic/recurrent PE was associated with RV hypertrophy, whereas RV dilatation resulting from acute PE or RV infarction was not. Furthermore, ventricular septal shift is commonly observed in acute PE and not in RV infarction. Moderate to severe RV hypokinesis after PE, with an RV/LV diastolic diameter ratio of N 1 on TEE, is associated with significantly greater in-hospital morbidity. In addition to assisting in the diagnosis of PE, TEE is useful in establishing alternative diagnoses such as aortic dissection, pericardial disease, hypovolemia, myocardial dysfunction/infarction, and valvular regurgitation, as well as in guiding resuscitation therapy in hemodynamically unstable patients [29, 30].
Initial treatment of PE may begin before a definitive diagnosis is made or even before the patient leaves the operating room, beginning with supportive therapy to stabilize the patient and minimize the effect of embolic occlusion. Vasopressors should be used to improve RV function and systemic vasoconstriction to maintain BP and CPP in the face of persistent obstruction to blood flow due to embolism. Noradrenaline may be the pressor drug of choice for the treatment of hypotension in patients with massive PE. The beneficial effects of norepinephrine may be primarily due to its α-1-mediated vasoconstriction, which increases BP and RV CPP, and also increases venous return. Moreover, stimulation of β1 by noradrenaline increases both RV and LV contractility and CO. Alternatives to noradrenaline are dopamine, epinephrine and dobutamine. However, dobutamine may cause undesirable peripheral vasodilation via a β2 mechanism and may also reduce arterial oxygenation by altering the V/Q relationship. Combination treatment with dobutamine and noradrenaline may be considered. Pulmonary vasodilators, such as inhaled nitric oxide, may be useful in lowering PA pressure, increasing CO, and improving RV function and pulmonary gas exchange in patients with PE, without significantly reducing systemic BP [31].
Pulmonary embolism occurs with some frequency in perioperative patients and should be included in the differential diagnosis of perioperative hypoxemia, hypotension, tachycardia, or acute end-tidal CO2 reduction. Hemodynamic instability is a major risk factor in patients with perioperative PE. Nevertheless, it is often possible to make at least a preliminary diagnosis of PE and even initiate effective treatment in the operating room or in the early postoperative period.
The study presents a rare but significant case of massive pulmonary embolism and perioperative cardiac arrest during nephrectomy due to malignancy, with infiltration of the inferior vena cava. The description of this incident constitutes an important contribution to the medical literature, emphasizing the need for rapid response and appropriate intervention in such clinical emergencies. The case of a patient undergoing nephrectomy for malignancy who experienced cardiac arrest during surgery due to a massive pulmonary embolus provides a detailed and instructive illustration of the situations that may occur during this type of procedure. The authors describe in detail the course of the surgery itself and the rescue intervention. Additionally, the literature review presents a variety of therapeutic approaches and methods for managing similar clinical situations, allowing for better understanding and preparation for similar incidents. The analysis of available literature also enables the assessment of the effectiveness of the interventions used and the identification of best practices in the area of saving the lives of patients in critical situations.
The conclusions of the article have significant practical significance for surgeons, anesthesiologists and medical personnel involved in surgical procedures, especially those related to the removal of malignant tumors. The presented case and literature review may contribute to a better understanding of the risks associated with such procedures and to improve.