- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 2: e1. DOI 10.35630/2024/14/2.205
Antitumor drugs are toxic to the cardiovascular system and directly affect cardiac function and anatomy. During the treatment of oncology, patients may experience complications in the form of asymptomatic myocardial dysfunction, severe heart failure, exacerbation of symptoms of coronary heart disease, thromboembolic complications, arterial and pulmonary hypertension, pericardial complications, valve defects and arrhythmias. A number of diagnostic tools are currently available to cardio-oncologists to detect cardiotoxicity, and the choice of imaging method depends on the availability of the specific diagnostic modality and its resolution. The main method for assessing the condition of the heart in cancer patients is echocardiography - a widely available method that enables to assess the structure of the heart and hemodynamics without exposing the patient to an additional dose of ionizing radiation. If imaging is insufficient, the recommended method for assessing cardiac structure and function is magnetic resonance imaging. Computed tomography of the chest, heart and coronary arteries is also used to diagnose complications of cancer treatment. In addition, nuclear medicine techniques, including gated radionuclide ventriculography, are also used to diagnose cardiotoxicity. Electroanatomical mapping systems combined with remote navigation are promising. Early detection of subclinical left ventricular myocardial dysfunction in cancer patients receiving potentially cardiotoxic drugs is advisable and aimed at secondary prophylaxis and prevention of mortality from cardiovascular diseases.
Keywords: cardio-oncology, echocardiography, computed tomography, magnetic resonance imaging, cardiac nuclear tomography, multimodal imaging, electromechanical myocardial mapping, navigation systems
Progress in the treatment of oncology made over the past 20 years has led to an increase in the survival rate of cancer patients. However, anticancer treatments are associated with significant cardiovascular toxicity, with heart disease potentially leading to premature death. This is due to therapeutic cardiotoxicity and increased severity of existing cardiovascular diseases. Cardiotoxic events may result from interactions between different therapeutic modalities. Such complications can manifest in the form of a number of cardiovascular disorders, including asymptomatic myocardial dysfunction, severe heart failure, coronary heart disease, thromboembolic conditions, pulmonary hypertension, pericardial complications, valvular defects, arrhythmias, etc. All possible complications of cancer therapy affecting cardiovascular system require imaging for diagnostic work, as well as monitoring of therapy. While standard techniques such as routine transthoracic echocardiography (TTE) may be sufficient for the initial assessment of a patient with symptoms suggestive of cardiac abnormalities, specialized assessment modalities, including nuclear imaging, are available to characterize the abnormalities in detail and improve patient management.
To evaluate coronary heart disease, echocardiography is often performed as an initial test to assess cardiac function and rule out other cardiac conditions that may be causing the patient's complaints. Normal resting echocardiographic studies cannot rule out underlying coronary artery disease because regional wall motion defects in the setting of coronary artery disease are observed only in critical ischemia or previous myocardial infarction. For unstable angina with suspected coronary heart disease, stress echocardiography with both pharmacological and physical stress is widely used in clinical practice. There is a wealth of data from large-scale multicenter studies of the effectiveness of stress echocardiography, which demonstrate well enough the safety and prognostic value of this method in clinical practice [1]. The results of stress echocardiography can be used as a precursor to coronary angiography.
Echocardiography is the first choice imaging modality for detecting myocardial dysfunction before, during and after cancer treatment [2, 3]. The Simpson’s biplane two-dimensional method is primarily used to assess left ventricular (LV) volumes and ejection fraction. For assessment of left ventricular ejection fraction (LVEF) by echocardiography, cancer therapy-related cardiac dysfunction is defined as a decrease in LVEF of more than 10 percentage points to a value below the lower limit of normal. If LVEF is decreased, re-evaluation is necessary to confirm impairment of LV systolic function and assess potential reversibility [4].
Diastolic dysfunction such as a prolonged phase of isovolumic relaxation, decreased peak flow velocity, the ratio of early peak flow velocity to atrial peak flow velocity, and decreased deceleration rate of early peak flow velocity may precede resting systolic dysfunction [5]. Stoddard et al. reported that prolongation of the isovolumic relaxation phase by Doppler echocardiography predicted systolic dysfunction in patients receiving doxorubicin [6]. Doxorubicin-induced reductions in ejection fraction of 10% to 55% were observed in 9 of 26 patients in this study, with an increase in isovolumic relaxation phase from 66 to 84 m/s after a cumulative doxorubicin dose of 100 to 120 mg/m2. However, the modest reproducibility of diastolic measurements by echocardiography limits its value, as diastolic measurements have varying levels of evidence for detecting signs of impaired cardiac function after cancer treatment [7].
While chemotherapy agents do not directly affect heart valves, valvular heart disease is often observed in patients with cancer due to radiation therapy, infective endocarditis, pre-existing valve disease, or secondary LV dysfunction. Approximately 10% of patients treated with radiation therapy will develop concomitant valvular disease during their lifetime. Since radiation-induced valvular disease has a distinct pattern of fibrosis and calcification of the aortic root, aortic valve leaflets, mitral valve annulus, and base and midportion of the mitral valve leaflets, echocardiography can differentiate it from rheumatic disease. In patients with Hodgkin's lymphoma, an association between cardiac radiation dose and the development of valvular abnormalities after treatment has been described, especially at doses >30 Gy [8]. However, since current protocols limit mediastinal radiation dose to 20 or 30 Gy, the risk of heart defect in cancer patients is only slightly increased compared with patients not treated with radiation therapy [9].
Echocardiography is also used in patients with arrhythmias to identify underlying structural heart diseases. In the case of atrial fibrillation, this imaging method, in particular, includes assessment of the size of the left and right atria, as well as assessment of the function of the tricuspid and mitral valves. In addition, echocardiography can evaluate indicators of increased ventricular filling pressure, which can also lead to atrial enlargement and therefore cause atrial arrhythmia. In the case of ventricular arrhythmias, echocardiography can reveal underlying pathologies, which may include myocardial scarring and hypertrophic or dilated cardiomyopathy. Similarly, echocardiography may be used when rare causes of ventricular arrhythmias are suspected, including cardiac amyloidosis, arrhythmogenic right ventricular cardiomyopathy, or cardiac sarcoidosis.
Over the past decade, indications for cardiac CT have expanded. Non-contrast CT scans are primarily used to quantify subclinical coronary atherosclerosis to improve risk prediction in relevant populations. Contrast-enhanced studies are often used to exclude coronary artery disease in patients with chest pain due to its high negative predictive value [10].
Coronary CT angiography enables to assess the amount of coronary plaques and the manifestations of cardiovascular diseases [11]. The major clinical indication for coronary CT angiography is due to its high negative predictive value for safely and effectively ruling out coronary artery stenosis in most symptomatic patients with a high likelihood of coronary heart disease. Following radiation therapy, there is a dose-dependent association with the development of vascular diseases. Traditional cardiovascular risk factors may even increase the risk of cancer therapy, with a latency period of 10 years after initial cancer therapy [12].
For asymptomatic patients, non-contrast cardiac CT for coronary calcium scoring can assess individual risk for future cardiovascular events. Assessment of coronary artery calcification using non-contrast cardiac CT is the best existing method for predicting asymptomatic patients [13]. Since patients after cancer treatment may be asymptomatic due to the neurotoxicity of radiation or chemotherapy affecting the patient's perception of angina, image-based assessment of subclinical atherosclerosis may improve individual risk analysis [14].
In the case of valvular heart disease, CT may be useful to evaluate the degree of calcification of the ascending aorta, which is often observed after mediastinal radiotherapy, and can significantly increase the surgical risk [15]. In addition, cardiac CT is preferred for evaluating pericardial changes in suspected pericardial disease that may follow radiation or chemotherapy or a combination of both. CT scan shows both calcification and thickening of the pericardium. In the presence of pericardial effusion, three-dimensional evaluation using CT can determine its volume and differentiate between hemorrhagic and serous effusions.
In case of rhythm disorders, indications for cardiac CT are limited. During scanning, atrial fibrillation and flutter significantly impair imaging. Likewise, CT scanning may be difficult if the patient is suffering from tachycardia. In contrast, if a stable heart rhythm is achieved, coronary CT angiography can be used to identify suspected coronary heart disease. In addition, cardiac CT provides three-dimensional imaging of the left atrium and pulmonary veins, which can be useful in planning electrophysiological studies, and its integration with radioscopic imaging can improve the efficiency of the procedure [16].
Over the past decades, MRI has become a powerful tool for cardiovascular imaging. It is increasingly used as a basis for the study of various diseases due to the wide variety of imaging capabilities that include quantification of right and left ventricular systolic function, assessment of wall motion abnormalities, and detailed tissue characterization (such as fibrosis, scarring). Other important advantages of MRI are the very high contrast of soft tissues and the absence of ionizing radiation for the patient. However, the main disadvantages of MRI are: strong magnetic fields, which often exlude imaging in patients with various cardiac devices, such as pacemakers, internal cardiac defibrillators, left ventricular assist devices, or mechanical heart valves [17].
Cardiac MRI can detect coronary heart disease and quantify the degree of ischemia with high diagnostic accuracy [18]. One of the ways to detect vascular changes at the subclinical stage is to assess pulse wave velocity (PWV). PWV is a marker directly related to vascular stiffness, which in turn allows to draw conclusions about the burden of atherosclerotic disease at an early stage [19]. After the administration of cardiotoxic chemotherapy such as anthracyclines, thoracic aortic PWV as an indicator of vascular stiffness increases steadily. This phenomenon has been demonstrated in patients with breast cancer, as well as possible reversibility of these early vascular changes after discontinuation of anthracyclines [20].
Cardiac MRI can evaluate early changes in the myocardium caused by cancer treatment. Parameters include, but are not limited to, assessment of edema, fibrosis, extracellular volume and scarring. Since these biomarkers generally indicate early changes in the heart before impairment of LV systolic function, assessment of these parameters is of high clinical relevance for monitoring myocardial health during cardiotoxic cancer treatment. Myocardial edema is considered one of the earliest surrogate markers of chemotherapy or radiation damage to the heart. In a study by Oberholzer et al., 28 patients receiving anthracycline-based chemotherapy underwent cardiac MRI [21]. Using a specific binary threshold, 33% and 49% of patients were diagnosed with myocardial edema after 1 and 4 months respectively.
Cardiac MRI is often cited as the reference method for quantifying LV volume, mass, and wall motion abnormalities [22]. However, MRI is not usually the first choice for risk stratification before initiating cardiotoxic cancer treatment. While echocardiography is usually performed first, MRI may be helpful in nondiagnostic studies, in patients with unclear right or left ventricular dilatation, or in those with suspected infiltrative heart disease. However, there are no data on whether MRI performed before cardiotoxic treatment can identify patients at higher risk.
Nuclear medicine, using various radiopharmaceuticals, has a long history of identifying, detecting, and treating myocardial cardiotoxicity.
As described above, LV dysfunction is a very common finding in patients suffering from cardiotoxicity. While techniques such as echocardiography and MRI are increasingly used to assess LV volume and systolic function, nuclear medicine techniques such as multigated radionuclide angiography in 2-dimensional (MUGA, also known as radionuclide ventriculography) or 3-dimensional imaging have already been used for this purpose for a long time. This method is highly reproducible and can also be used in patients with cardiac devices (incompatible with MRI) or in patients in whom echocardiography is difficult (for example, obese patients). Numerous studies have been published that demonstrate the value of this method [23, 24].
In nuclear cardiology, positron emission tomography (PET) and single photon emission computed tomography (SPECT) are still most often performed to assess myocardial perfusion, especially for such clinical issues as the diagnosis of coronary heart disease, the hemodynamic significance of coronary stenosis, to adjust treatment (for example, the degree of myocardial ischemia) and for risk stratification of patients [25].
PET allows for absolute quantitative assessment of regional myocardial blood flow. As for myocardial perfusion imaging, most studies have focused on patients undergoing radiation therapy for breast or chest cancer. In a study by Gayed et al. 51 patients with esophageal cancer were examined for myocardial perfusion defects [26]. A total of 26 patients underwent radiation therapy, and 54% of them had impaired myocardial perfusion. In the non-irradiated group (n=25), only 16% had perfusion defects. LVEF did not differ between the two groups. While PET studies of myocardial perfusion imaging in patients undergoing cardiotoxic treatment are few and have shown contradictory results, an experimental study in dogs exposed to local cardiac irradiation clearly demonstrated myocardial perfusion defects on PET/CT with N-13 ammonia [27]. Interestingly, hearts of irradiated dogs showed an initial increase in perfusion after 3 months, decreased perfusion after 6 months, and a perfusion defect after 12 months. In addition, LVEF decreased over time in exposed dogs.
The most commonly used tracer for PET is fluorodeoxyglucose F-18 (FDG). The accumulation of this tracer in the cell is directly proportional to the efficiency of the functioning of the protein glucose transporter and correlates with the activity of hexokinase II, an enzyme that exchanges the hydroxyl group of glucose for the phosphate complex of ATP. The phosphorylated metabolite of 18F-fluorodeoxyglucose loses its ability to be transported across the cell membrane and remains intracellular. Thus, FDG, unlike ordinary glucose, when entering the cell, participates only in the initial stages of glycolysis, and therefore does not break down, which leads to its progressive accumulation in the cell (the “metabolic trap” phenomenon). To visualize myocardial inflammation, glucose uptake by cardiomyocytes is minimized using various strategies: a high-fat, low-carbohydrate diet before F-18 FDG PET, prolonged fasting, or pre-injection of heparin before F-18 FDG administration (the latter stimulates various lipases, which increases the level of free fatty acids in the blood). If FDG F-18 is administered with such a drug, the physiological accumulation of FDG F-18 in the heart is minimized, and inflammation foci can be visualized with high contrast. In a preclinical study, mice treated with sunitinib the tyrosine kinase inhibitor showed increased cardiac glucose uptake 48–72 hours after start of treatment [28]. In addition, the animals showed a decrease in LVEF as a sign of incipient heart failure. Also, ultrastructural changes were detected, such as lipid accumulation and mitochondrial dysfunction. Accumulating evidence suggests that anthracycline chemotherapy alters myocardial glucose metabolism as demonstrated by F-18 FDG PET. In preclinical and retrospective clinical studies, increased cardiac F-18 FDG accumulation has been observed as a potential early sign of cardiotoxicity [29, 30]. In addition to chemotherapy-induced cardiotoxicity, a preclinical study in dogs described radiation-related myocardial metabolic changes [31]. Accordingly, a retrospective clinical study found increased cardiac F-18 FDG uptake after stereotactic irradiation of lung tumors close to the heart [32]. Cardiac uptake of F-18 FDG correlates with cardiac radiation dose, possibly indicating radiation-induced myocardial damage.
The Noga XP navigation system is designed for more accurate delivery of cells to ischemic myocardium. The operating principle of the system is magnetic navigation. The system makes it possible to perform volumetric reconstruction of the left ventricle and at the same time determine the viability of the myocardium [33-35], and using a special injector, introduce cellular material into the desired segment.
The reconstruction algorithm finds a boundary ellipsoid from the available samples and then projects the samples onto this ellipsoid. This ellipsoid does not depend on the spread of the points taken, i.e. the area where the points are close together is just as important as the area where the points are far apart.
To determine the contour, it is necessary to take at least 4 points (notably not located in the same plane). In addition to the location, the local activation time of each point is recorded. These values determine the color interpolation: early activation is indicated in red, late activation is in purple. As new points are taken, the early and late activation zones are rebuilt (updated). The spaces between the taken points are filled with color, and the color is interpolated according to the color scale.
The method of mapping and the color shading are based on the principle of triangulation, when a so-called lattice of triangles is initially constructed. The distance between nodal points plays an important role. The reliability of the reconstructed surface and its color decreases if the distance between the nodal points increases. The electrophysiologist himself chooses the scale or threshold for map coloring – the lower the threshold, the more “voids”, i.e. the map turns out to be the most accurate and most detailed.
Points are taken in sinus rhythm under conditions of stable catheter location, stable local activation time (LAT), stable cycle length (LS no longer than 3 mm) and in the absence of ST segment elevation on the unipolar reference channel. Thus, an electroanatomical map of the left ventricle is built. This type of map displays the geometry of the left ventricle and the electrical activation sequence of the ventricle. Fibrous rings of the aortic and mitral valves are indicated as anatomical landmarks. The system automatically determines the apex of the left ventricle as the most distant point from the aortic valve. If necessary, the localization of the apex is changed manually. The electroanatomical map is then switched to unipolar voltage and mechanical (LLS) mode. On the unipolar voltage map, myocardial segments with a spike amplitude below 7 mV are considered a scar. On the mechanical map (LLS), areas with wall motion amplitude lower than 12% of the maximum are considered scar or insufficiently vascularized myocardium. When comparing the voltage unipolar and mechanical maps, myocardial segments are determined that are viable, but are in a state of ischemia and the contraction amplitude of which is significantly reduced. These are zones of the so-called hibernated myocardium. They are the target for cell therapy. Fig. 1
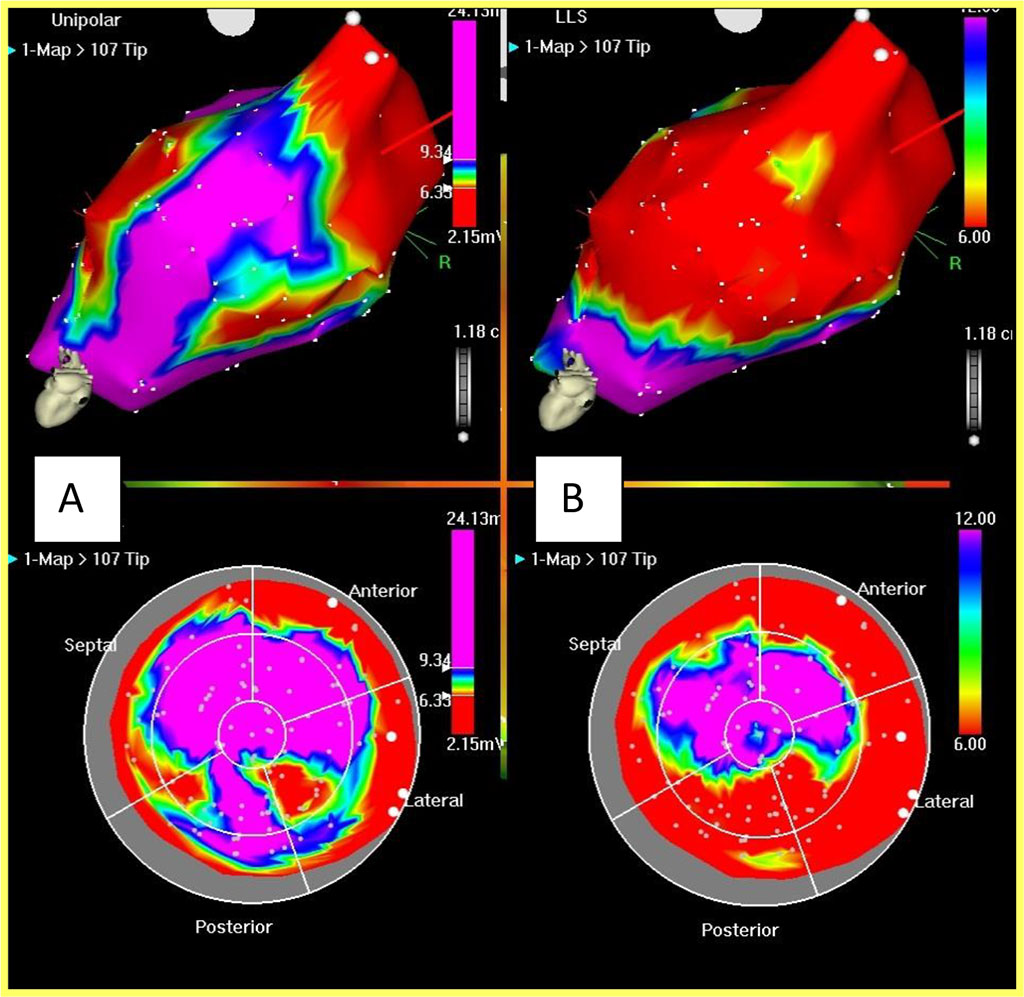
Fig. 1. Unipolar voltage and mechanical maps of the left ventricle
The unipolar voltage map (A) allows to identify areas with low-amplitude potentials (scar), in red on the map. Violet zones are zones of viable myocardium. High-amplitude electrical activity is recorded in these zones. The mechanical map (B) reflects the amplitude of wall motion. Red zones are poorly contracting or non-contracting segments. Violet zones are well-contracting myocardium. The red zones on the 2 types of maps may not coincide, because in the areas of hibernated myocardium, high-amplitude electrical activity is recorded, but they contract poorly. These areas are the target for injection.
After completion of the left ventricular mapping process, the mapping catheter is replaced with a Myostar catheter (Biosense Webster, USA) intended for intramyocardial administration of active agents. The length of the retractable needle is preliminarily adjusted using an aortic arch simulator (the length of the retractable needle is ½ the thickness of the myocardium). Injections of mesenchymal autologous bone marrow stem cells are performed into areas of hibernated myocardium, while avoiding injections into the area of the apex of the mitral valve due to the high risk of perforation, and into the area of recording His bundle potentials due to the risk of blockade development.
The search for an ideal electroanatomical mapping system combined with remote navigation is still ongoing. An ideal system would combine ease of use, high speed, and the ability to simultaneously display multiple arrhythmias, provide reliable information about the mechanism lying at the root of arrhythmia, and identify adequate targets for ablation.
There is a large arsenal of imaging techniques that can be used to monitor the cardiotoxic effects of anticancer treatments. The great advantage of echocardiography is that it is widely available and non-invasive. CT is very suitable for assessing coronary anatomy. MRI is useful in detecting abnormal myocardial perfusion, scarring, and early signs of myocardial damage such as edema and incipient fibrosis. Nuclear medicine is able to visualize molecular signals of myocardial damage, such as perfusion abnormalities, cell death, and changes in metabolism and innervation. Most studies and imaging techniques focus on abnormalities of LV function and wall motion and, unfortunately, in the late stages of the process of cardiotoxicity. Recently, however, new approaches have been explored to identify early prognostic factors before the onset of LV dysfunction, in particular using MRI and nuclear medicine techniques. When using new treatments (for example, checkpoint inhibitor therapy), careful monitoring of patients is necessary to detect possible cardiotoxicity. The quality of modern electroanatomical mapping systems is quite high, and yet new tools and devices are appearing that facilitate the process of understanding arrhythmias and thereby simplify their elimination. Further prospective studies are needed to explore surrogate markers of incipient cardiotoxicity.
The authors have no conflict of interests to declare.