- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 2: e1. DOI 10.35630/2024/14/2.203
The purpose of this research was to study the characteristics of the immunohistochemical reaction of lymph nodes after intralymphatic immunostimulation with acid peptidoglycan in a rat model.
Material and Methods: Immunostimulation was modeled in groups by introducing APG with a molecular weight of 1000–40000 kDa at a dose of 0.3 U. The drug was administered intramuscularly in the comparison group (n=30) and intralymphatically in the main group (n=30), on days 1, 2, 3, 8, 9, 10 of the experiment. Hyaluronidase was used as a lymphotropic substance at a dose of 0.07 U. We used Bcl-2 rabbit antibodies, сlone: SP66 (Roche, Switzerland) and CD-3 antibodies (PrimeBioMed, Russia). The UltraVision Quanto Detection System HRP Polymer (ThermoFisher, USA) was used as a detection system.
Results: Intralymphatic administration of APG gives an immunostimulating effect on the third day from the start of administration. Meanwhile, with intramuscular immunostimulation, the effect occurred only on the seventh day of the experiment (p≤0.05). Immunohistochemical studies using Bcl-2 and CD-3 showed the effectiveness of APG as an immunostimulator, which was confirmed by increased expression of CD-3+, while an increase in the pool of Bcl-2+ cells indicated strengthened barrier and anti-apoptotic properties of lymph nodes. The duration of the immunostimulating effect depended on the route of APG administration. With the lymphatic route of administration, the effect occurred on the third day, with the intramuscular route – on the seventh day from the start of administration of the immunostimulant (p≤0.05).
Conclusion: An APG based drug stimulates the expression of genes for signaling receptors of immune effectors, which increases their sensitivity to pathogens of various natures and elicits an adequate immune response in the body.
Keywords: acid peptidoglycan, immunostimulation, lymph nodes, intralymphatic administration, immunohistochemistry, BCL-2, CD-3
In the last decade, immunomodulators have proved to be promising therapeutic agents in the treatment of infectious, autoimmune and oncological diseases, as well as for the prevention of organ transplant rejection. The therapeutic effects of immunomodulators can be achieved by enhancing or suppressing the activity of immune cells [1-5].
In entering this study, we hypothesized that immunomodulators that can trigger and activate the immune response at the surgical site would be likely to reduce surgical site infection. This hypothesis had been confirmed by previous studies. For example, Mahmud F. et al., (2022) used three immunomodulators – fMLP (formylmethionine-lysine-proline), CCL3 (MIP-1α) and LPS (lipopolysaccharide) – based on their reported ability to induce immune responses in a model of surgical wound infection by Pseudomonas aeruginosa. The findings indicated that single, low-dose topical treatment with these immunomodulators significantly enhanced proinflammatory responses in infected and uninfected surgical wounds and was as effective as (or even better than) a potent prophylactic antibiotic (tobramycin).
These studies showed that immunomodulators did not have an adverse effect on tissue regeneration and wound healing processes. The authors noted better healing of both infected and non-infected wounds [6].
Taken together, the data suggest that harnessing the power of the innate immune system through immunomodulators can significantly improve infection control and potentially promote healing. It has been suggested that treatment with immunomodulators (alone or in combination with prophylactic antibiotic therapy) during surgery may have therapeutic potential in the control of SSIs [7].
Since the lymphatic system is the main reservoir of immune cells, a modern strategy may be to target immunomodulators to gut-associated lymphoid tissue (GALT) [8-13]. In recent years, lymphogenous methods of administering immunomodulators have become one of the most promising in clinical and experimental lymphology. These methods have been applied in surgery, traumatology, gynecology, dermatovenerology, phthisiology and many other specialties [14-18].
In the course of evolution, multicellular organisms had a need to create a mechanism for recognizing “friend or foe”. One of the important components of the innate immune system is the peptidoglycan recognition system using peptidoglycan recognition proteins [19-23]. Peptidoglycan is part of the cell wall of both gram-positive and gram-negative bacteria, being a good target for recognition by components of the innate immune system [24-28]. Peptidoglycan recognition protein family includes secreted proteins that may be involved in mechanisms of immune response that are more complex than simple ligand–receptor interactions [29-35].
In addition to bactericidal properties, mammalian peptidoglycan recognition proteins may exhibit immunomodulatory properties. For example, the PGLYRP-3 protein enhances phagocytosis and the proinflammatory response to peptidoglycan in THP-1 cells [36, 37].
It should be noted that different molecules will produce very different responses in different tissues and cells under different conditions. However, with the exception of very few but notable cases (eg, neutrophil priming in the bone marrow, brain development and behavior, intestinal inflammation, and susceptibility to infection), the role of peptidoglycan in development/physiology/pathology in most organs and tissues remains to be elucidated [38]. Analysis of individual peptidoglycans, together with genetic profile, immune tone, and known host health/disease status, may also reveal an entirely new system for modulating health and disease.
The purpose of this research was to study the characteristics of the immunohistochemical reaction of lymph nodes after intralymphatic immunostimulation with acidic peptidoglycan in a rat model.
The study was conducted on 60 male Wistar rats of mature age weighing 150-170 g. The experiment took place in a certified laboratory in compliance with the “European Convention for the Protection of Vertebrate Animals Used for Experimental or Other Scientific Purposes” [Directive 2010/63/EU]. The animals were divided into 2 groups – the main group and comparison group of 30 animals each.
Immunostimulation was modeled in groups by introducing APG with a molecular weight of 1000–40000 kDa at a dose of 0.3 U. The drug was administered intramuscularly in the comparison group and intralymphatically in the main group, on days 1, 2, 3, 8, 9, 10 of the experiment. Hyaluronidase was used as a lymphoprotective substance at a dose of 0.07 U. The animals were removed from the experiment on days 1, 3, 7, 14 and 30. Collection, fixation of mesenteric lymph nodes and production of paraffin blocks were carried out according to the generally accepted method of working with lymphoid organs. We studied the expression of: Bcl-2, an intracellular protein factor that suppresses apoptosis in many cellular systems, including lymphohematopoietic and neuronal cells, regulates cell death by controlling the permeability of the mitochondrial membrane; CD-3, a multiprotein complex on the surface of T-lymphocytes, which is the main coreceptor of the T-cell receptor. Bcl-2 rabbit antibodies, сlone: SP66 (Roche, Switzerland), were diluted at 1:200 in antibody diluent; CD3 rabbit antibodies (PrimeBioMed, Russia) were diluted at 1:200. Sections were incubated with primary antibodies (50-100 μl per section) for 1 hour at room temperature in a humid chamber, and then washed with PBS-T buffer 3 times for 2 minutes. The UltraVision Quanto Detection System HRP Polymer (ThermoFisher, USA) was used as a detection system in accordance with the manufacturer’s instructions, namely: 2 drops of linker were applied to the section for 10 minutes, washed with PBS-T buffer 3 times for 2 minutes, then 2 drops of the conjugate with HRP polymer were applied per section for 10 minutes and washed with PBS-T buffer 4 times for 2 minutes. To visualize nuclei, we used Mayer's hematoxylin (BioVitrum, Russia) according to the manufacturer's instructions. Lymph nodes of intact rats (n=12) were used as a control.
Morphometric studies were carried out using the Morpholog computer program. Linear lymph node measurements were recorded by creating a “distance object” between two points. The sectional areas of the structural components of the lymph nodes (cortical, medulla and paracortical zone) were determined by the “masking” method. Morphometric calculation was carried out in six fields of view of each section, six sections of each object were analyzed.
Comparison of quantitative indicators in the study groups was carried out using the Wilcoxon-Mann-Whitney U test. The critical level of significance when testing statistical hypotheses was taken equal to 0.05. Statistical analysis was carried out using the Statistica v.6.0 program.
The analysis of the immunohistochemical reaction of the mesenteric lymph nodes on the days 3, 7 and 14 revealed the presence characteristic differences in the groups after intralymphatic and intramuscular administration of APG. Immunohistochemical changes are briefly described in the series of figures below. Fig. 1-8.
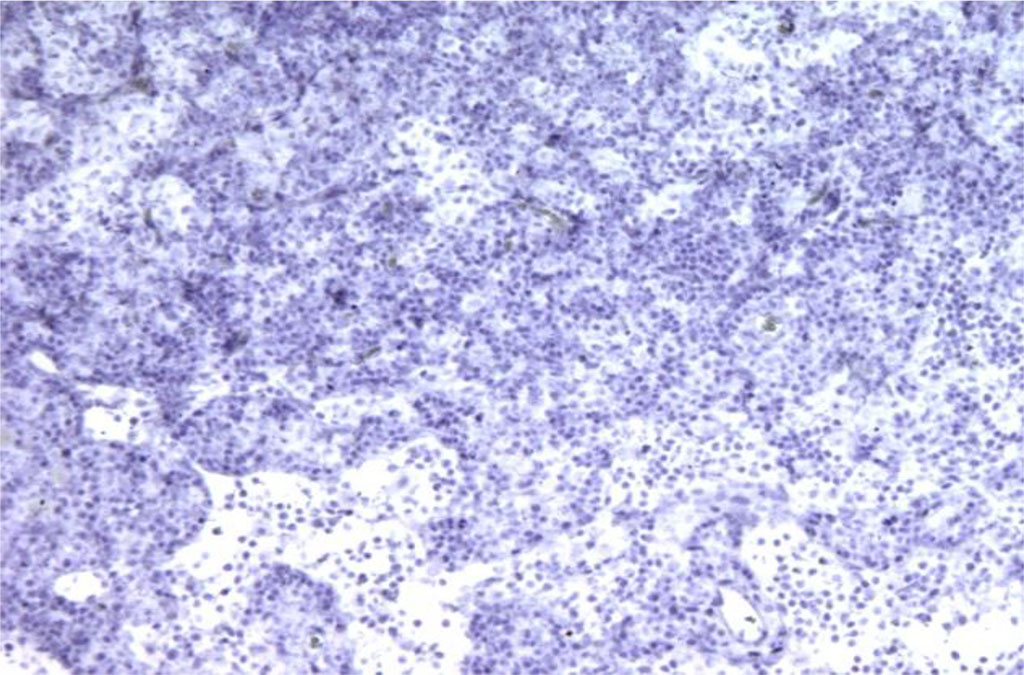
Figure 1. Rat lymph node. Morphological picture on the 3rd day of intramuscular administration of APG. Bcl-2 expression is absent. X100
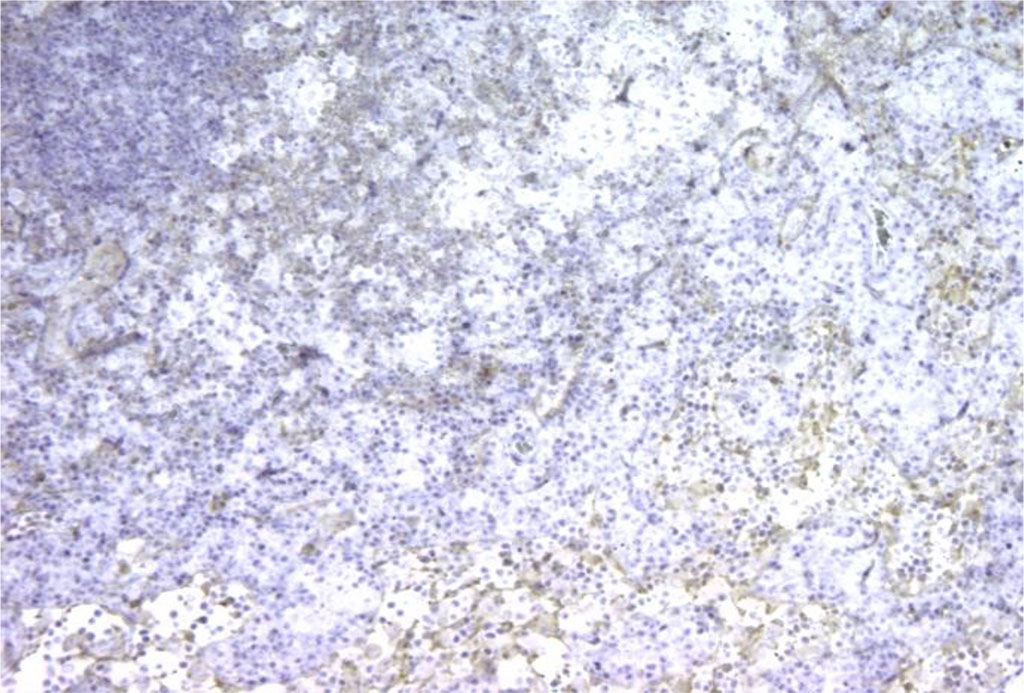
Figure 2. Rat lymph node. Picture on the 3rd day of intralymphatic administration of APG. The appearance of Bcl-2+ cell expression around the “light centers”. X100
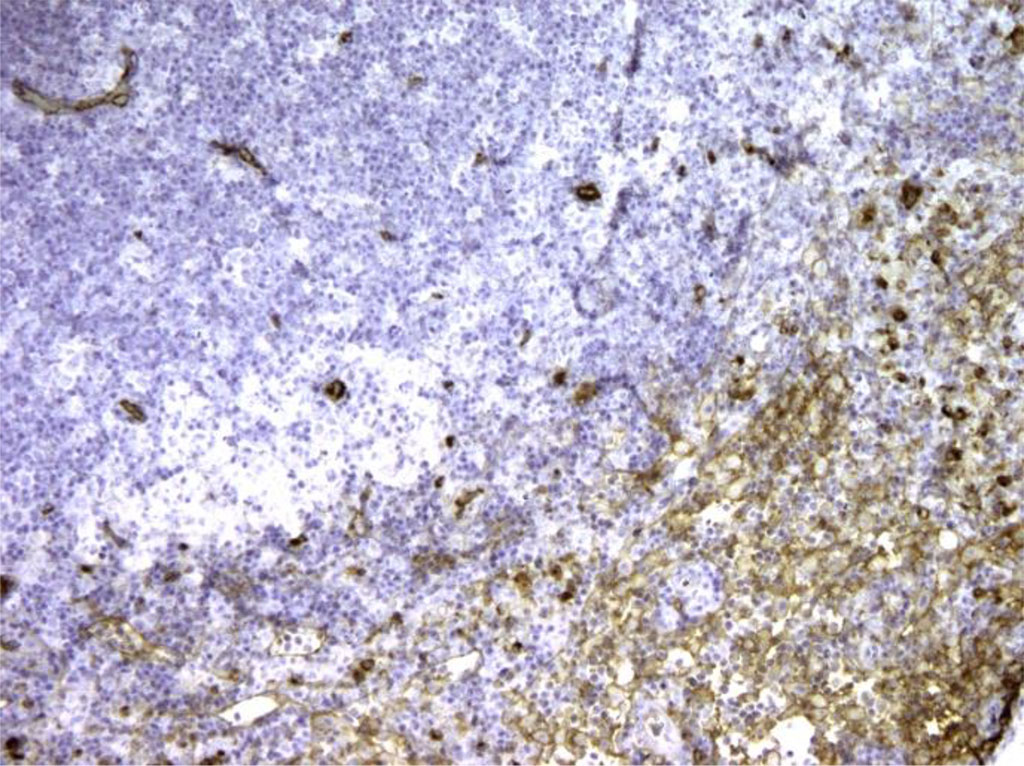
Figure 3. Rat lymph node. Morphological picture on the 7th day of intramuscular immunocorrection with APG. Bcl-2+ cells are located around light (germinal) centers, which suggests that antibodies bind to T cells. X100
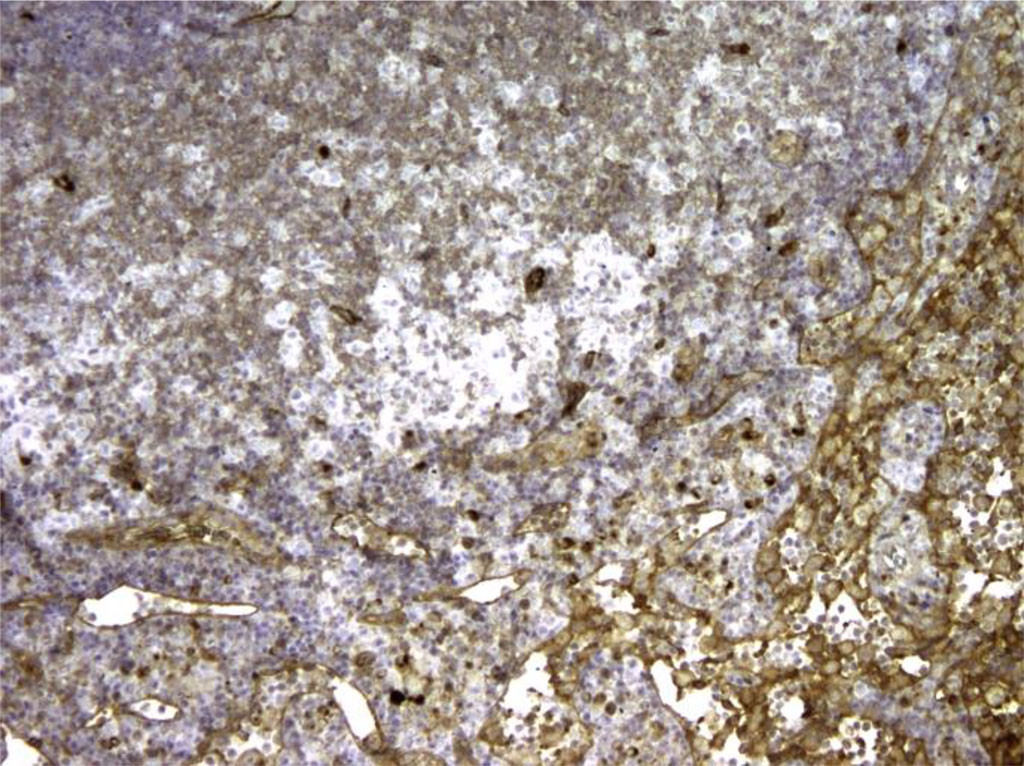
Figure 4. Rat lymph node. Morphological picture on the 7th day of intralymphatic immunocorrection with APG. Expression of Bcl-2+ in lymph node tissue. X100
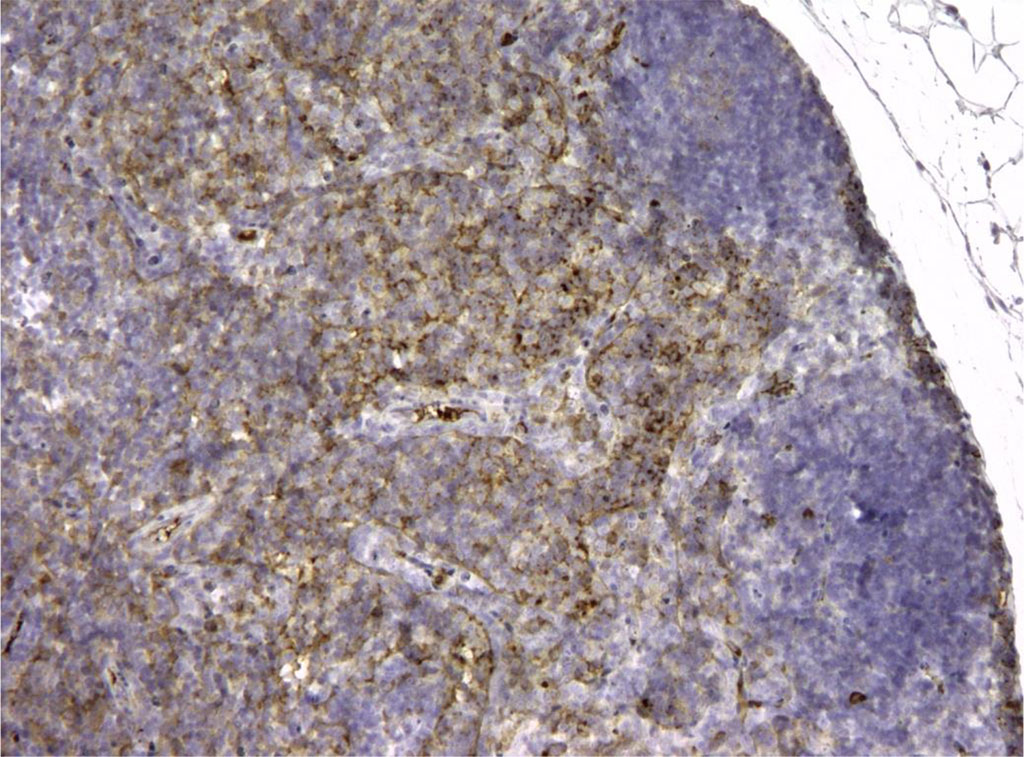
Figure 5. Rat lymph node. Morphological picture of the 3rd day of intramuscular administration of APG. In the “light centers” there are single CD3+ elements, that is, not fully differentiated lymphocytes. X100
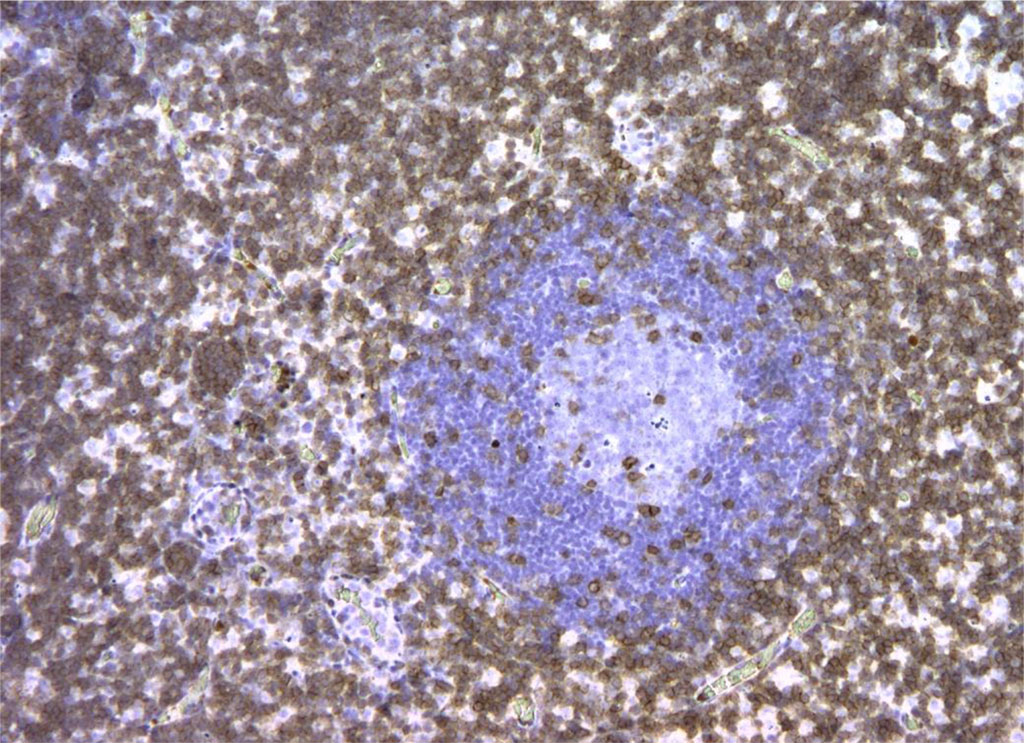
Figure 6. Rat lymph node. Picture on the 3rd day of intralymphatic administration of APG. CD3+ reaction around the “light centers” of 90% of cells. X100
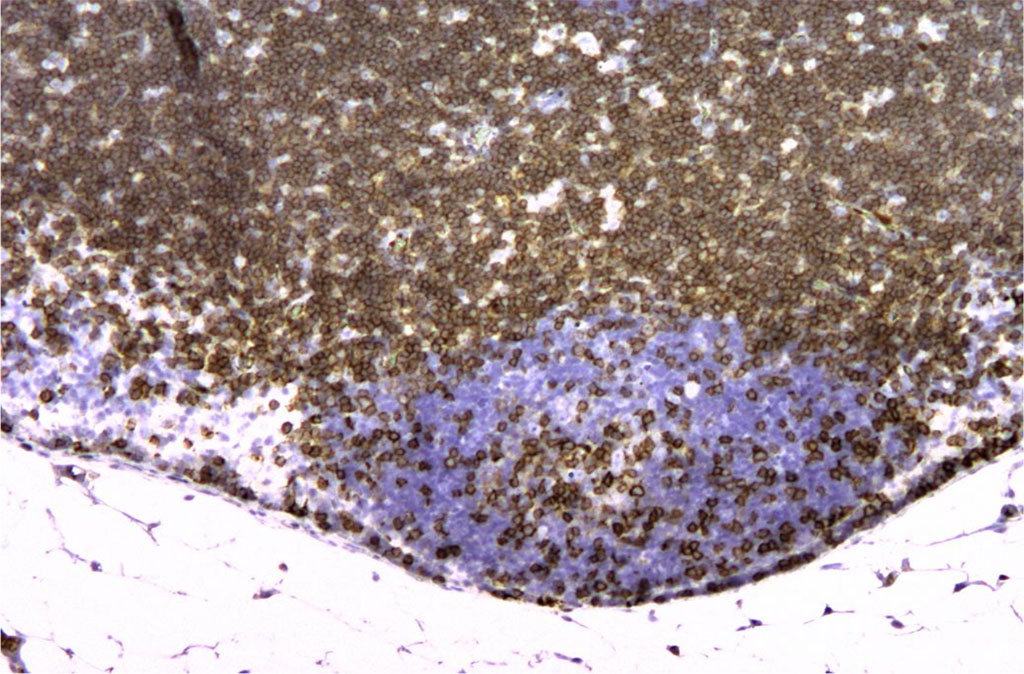
Figure 7. Rat lymph node. 7th day of intramuscular administration of APG. CD3+ staining is present mainly in the cortex. X100
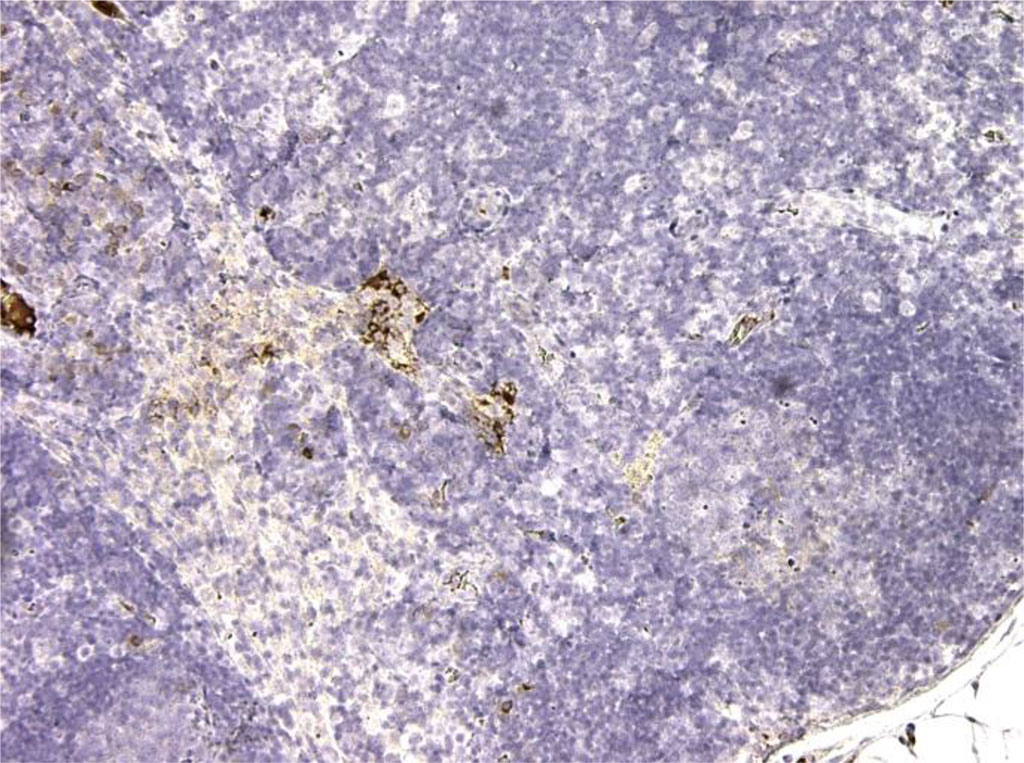
Figure 8. Rat lymph node. 7th day of intralymphatic administration of APG. CD3+ is observed in all T cells of the medulla and cortex. About 90% of cells. X100
Changes in the optical density of Bcl-2 and CD-3 immunopositive cells, as well as the relative area of expression of Bcl-2+ and CD-3+ cells in the groups had their differences in the timing of the onset of the immunostimulating effect. Tab. 1.
Table 1. Immunohistochemical indicators in study groups (Me [min; max]; M±sd)
Indicator |
Control (n=12) | Main group (n=30) | Comparison group (n=30) | ||
| Day 3 | Day 7 | Day 3 | Day 7 | ||
| Optical density of Bcl-2 immunopositive cells, au | 0.16±0.03 [0,12;0,19] | 5,66±0,02* [0,76;8,23] | 7,34±0,98 [2,34;9,34] | 0, 12±0,03 [0,11;0,17] | 6,03±0,34* [3,22;7,66] |
| Relative area of expression of Bcl-2 immunopositive cells, % | 12,65±0,34 [7,33;14,23] | 83,65±3,42* [76,34;93,23] | 87,45±3,22 [76,45;98,56] | 10,98±0,32 [8,32;14,10] | 76,45±4,77* [67,8;87,34] |
| Optical density of CD-3 immunopositive lymphocytes, au | 1,03±0,77 [0,67;1,99] | 2,27±0,44* [1,74;4,23] | 3,45±0,32 [1,22;4,33] | 0,55±0,07 [0,31;0,79] | 2,89±0,34* [1,45;3,24] |
| Relative area of expression of CD-3 immunopositive lymphocytes, % | 15,94±2,96 [8,14;22,04] | 77,04±3,21* [64,21;89,07] | 88,03±1,12 [5,43;10,06] | 14,89±2,67 [6,45;19,4] | 94,15±8,76* [86,65;97,53] |
* – reliability of changes in values at р≤0,05
Morphometric analysis in the group with intralymphatic administration of APG on the 3rd day revealed a significant increase in the density of lymphatic nodules per unit area of the sample, as well as the size of germinal centers, which increased by 36.4±2.1% and 32.1±1.6 % respectively (p≤0.05). Lymphatic nodules appeared not only in the cortex, but also in the medulla. There was a slight increase in the average size of lymph nodes, which was within the statistical error. Lymphatic cells filled the lumen of the follicles. On the 30th day, the density of lymphatic nodules and the size of germinal centers decreased by more than a third and amounted to 19% and 15%, respectively. In the control group, an increase in the studied parameters was noted on the 7th day of the experiment. A growth of lymph nodes was found to be 25.1±2.2%, germinal centers and the density of lymphatic nodules increased by 28.2±0.3% and 26.1±0.7%, respectively. On the 30th day, the above changes virtually returned to the initial values. Fig. 9.
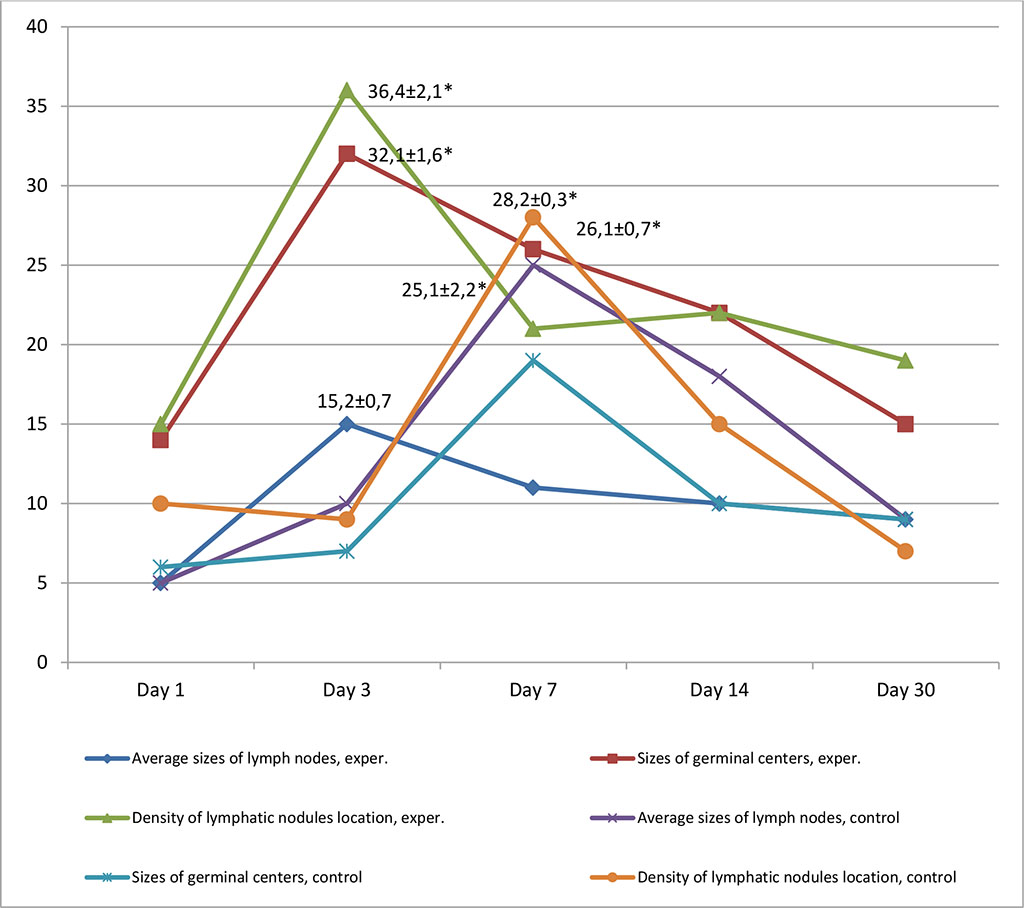
Figure 9. Dynamics of morphofunctional changes in lymph nodes with inralymphatic and intramuscular administration of APG at different observation dates (%); * - differences statistically significant at p <0.05
Thus, based on the above, we can conclude that intralymphatic administration of APG produces an immunostimulating effect on the third day from the start of administration. Meanwhile, with intramuscular immunocorrection with APG, the effect occurred only on the seventh day of the experiment (p≤0.05).
Immunohistochemical studies using Bcl-2 and CD-3 showed the effectiveness of APG as an immunostimulator, which was confirmed by increased expression of CD-3+, while an increase in the pool of Bcl-2+ cells indicated strengthened barrier and anti-apoptotic properties of lymph nodes. The duration of the immunostimulating effect depended on the route of APG administration. The effect occurred on the 3rd day from the start of administration of the immunostimulant by the lymphatic route of administration, and on the 7th day – by the intramuscular route. An APG based drug stimulates the expression of genes for signaling receptors of immune effectors, which increases their sensitivity to pathogens of various natures and elicits an adequate immune response in the body.