- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 2: e1. DOI 10.35630/2024/14/2.222
Using an experimental rat model, we analyzed the morphofunctional state of mesenteric lymph nodes after intralymphatic administration of various immunostimulants.
Material and Methods: The effect of four drugs was studied on 100 male rats: acid peptidoglycan, azoximer bromide, hexapeptide (arginyl-alpha-aspartyl-lysyl-valyl-tyrosyl-arginine) and aminodihydrophthalazinedione sodium. We studied morphometrically the average size of lymph nodes, the size of germinal centers, the density of lymph nodes, and calculated the mitotic activity index and migratory activity index.
Results: All experimental groups showed changes characterized by morphological restructuring of the lymph nodes, which was accompanied by an increase in their reactivity and provided an immunostimulating effect. In a dynamic study of the condition of the lymph nodes during the intralymphatic administration of various immunomodulators, it turned out that changes occur at different times. With the introduction of APG, the greatest structural changes occurred on the day 3 of the experiment. In the case of intralymphatic administration of AB, peak changes occurred on days 7-14 from the start of administration. In the third and fourth groups, using HP and ADPS, structural restructuring of the lymph nodes occurred on days 14-30.
Conclusion: When extrapolating the data obtained to the clinical plane, we can conclude that intralymphatic administration of APG can be recommended in the acute period of the disease, intralymphatic administration of AB - in the case of subacute course of the disease, whereas the use of HP and ADPS will be effective during the period of convalescence.
Keywords: immunotherapy, immunostimulation, immune regulation, lymph nodes, lymphatic transport, immunomodulators
The lymphatic system is a major component of the immune system and acts as a secondary circulatory system to drain excess fluid, proteins, and waste products from the extracellular space into the vascular system [1-5]. Lymphatic vessels have been used as potential drug delivery vehicles because certain lipophilic compounds, such as long-chain fatty acids, triglycerides, cholesterol esters, fat-soluble vitamins, and some xenobiotics, are transported through these channels [6-11].
The lymphatic system is difficult to access with conventional intravenous drug administration, limiting the amount of drug that reaches the lymphatic tissue [12-15]. Various factors that determine the entry of particles into the lymphatic vessels include: size, composition, dose, surface charge and molecular weight, etc. Particle size is the main factor. For example, there is an optimal range for lymphatic uptake of subcutaneously injected particles: particles >100 nm remain largely confined to the injection site, particles of 10–80 nm are well absorbed by lymphatic vessels, and small particles and molecules (<20 kDa) are absorbed primarily by rich capillary networks that drain into the systemic circulation [16-19].
In the last decade, immunomodulators have begun to be used as promising therapeutic agents in the treatment of infectious, autoimmune and oncological diseases, as well as for the prevention of organ transplant rejection. The therapeutic effects of immunomodulators can be achieved by enhancing or suppressing the activity of immune cells [20-22].
Thus, the development of lymph node-targeted drug delivery represents an important milestone and has the potential to improve the effectiveness of immune therapies. Targeted drug delivery also allows for reduced systemic dosage with a corresponding reduction in off-target toxicity [23]. Successful targeted delivery of immunoregulatory molecules to the lymphatic system has enormous potential for use in the treatment of a wide range of immune-mediated diseases.
The purpose of the study was a dynamic assessment of the morphofunctional state of the lymph nodes during intralymphatic immunostimulation with various drugs.
The study was conducted on 100 male Wistar rats of mature age weighing 150-170 g. The experiment took place in a certified laboratory in compliance with the “European Convention for the Protection of Vertebrate Animals Used for Experimental or Other Scientific Purposes” [Directive 2010/63/EU]. All animals were divided into 5 groups – a control one and four experimental groups of 20 animals each. Immunostimulation modeling in experimental groups was carried out using intralymphatic administration of various immunomodulators. In the first group, acid peptidoglycan (APG) with a molecular weight of 1000-40,000 kDa was used once a day at a dose of 1 IU. In the second group, azoximer bromide (AB) was used at a dose of 0.03 mg/day; the drug was administered once a day for 3 days, then every other day. In the third group, hexapeptide (HP - arginyl-alpha-aspartyl-lysyl-valyl-tyrosyl-arginine) was administered once a day at a dose of 0.7 μg/kg. In the fourth group, aminodihydrophthalazindione sodium (ADPS) was used at a dose of 2 mg twice a day. All received immunostimulants had official approval for use in clinical practice in Russia.
Intralymphatic injections of immunomodulators were performed subcutaneously into the projection of the lymphatic collectors of the hind limb for 14 days. Hyaluronidase was used as a lymphotropic substance at a dose of 0.07 IU; physiological solution was used as a solvent. In the control group (5th), animals were injected with a lymphotropic substance without immunomodulator.
Animals were removed from the experiment on days 1, 3, 7, 14 and 30. The object of the study was mesenteric lymph nodes.
Details of the histological structure of the lymph nodes were examined using a hardware-software complex, including a Leica MZ 12.5 stereomicroscope, a Pixera television camera, a DM-1000 Leica microscope and the Morpholog computer program. Linear lymph node measurements were recorded by creating a “distance object” between two points. The cross-sectional areas of the structural components of the lymph nodes (cortical, medulla and paracortical zone) were determined by the “masking” method. Morphometric calculation was carried out in six fields of view of each section, six sections from each object were analyzed.
Calculation of the mitotic activity index (Imit). On one section, we took into account from 3 to 6 germinal centers in which the number of lymphoid cells with mitotic figures was counted. The ratio of the number of mitoses to the number of examined secondary follicles is mitotic activity index of a given node (Antropova Yu.G., 1991). Formula for calculating Imit:
Calculation of the migratory activity index (Imig) involves calculating the ratio of the total number of lymphocytes located in the lumen of postcapillary venules (PCV) and lymphocytes adhering to the PCV wall to the number of examined PCVs in a given section. Formula for calculating Imig:
Comparison of quantitative indicators in the study groups was carried out using the Wilcoxon-Mann-Whitney U test. The critical level of significance when testing statistical hypotheses was taken equal to 0.05. Statistical analysis was carried out using the “Statistica” v.6.0 program.
The morphological structure of the mesenteric lymph nodes at different times after lymphotropic administration of immunomodulators retained its typical structure. Most of the section area of the lymph node in experimental animals is represented by the cortex; the boundary between the cortex and medulla was clearly visible.
In the first group with intralymphatic administration of APG, on the day 3, there was a significant increase in the density of lymph nodes per unit area of the sample, as well as in the size of germinal centers, which increased by 36.4±1.2% and 32.3±0.7%, respectively (p≤0.05). Lymphatic nodules appeared not only in the cortex, but also in the medulla. There was a slight increase in the average size of lymph nodes, which was within the statistical error. Lymphatic cells filled the lumen of the follicles. Fig. 1.
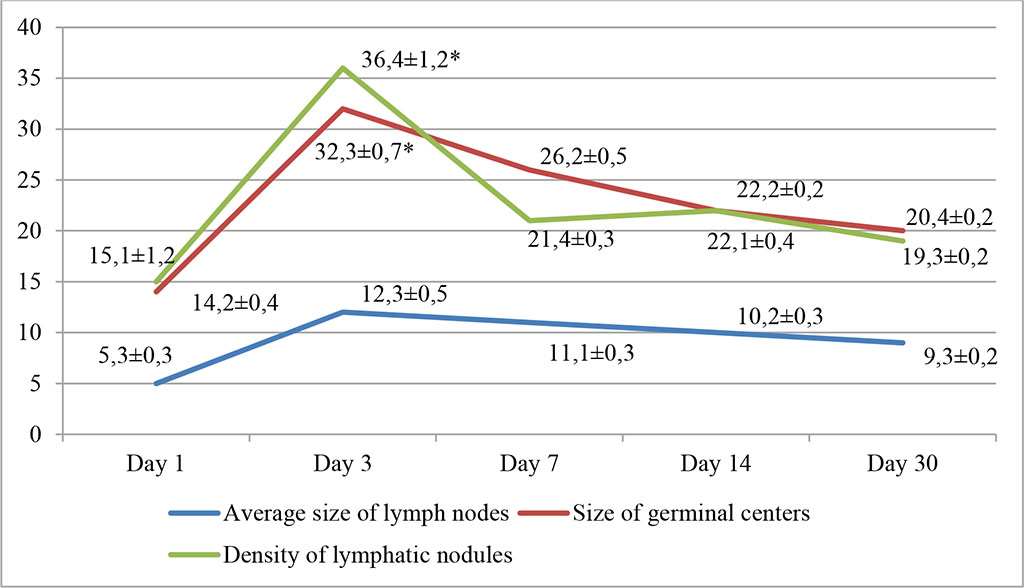
Fig. 1. Dynamics of morphofunctional changes in lymph nodes during intralymphatic administration of APG at different observation dates (%); * - differences are statistically significant at p<0.05
An increase in follicle size was noted; lymphoblasts and small lymphocytes predominated in the population of lymphoid cells, with smaller numbers of large lymphocytes and reticulocytes. An increase in the density of cells in the subcapsular zone of the lymph nodes was noted. The relative number of lymphoblasts exceeded the control values by 17.4% (day 3) and 14.4% (day 7). Subsequently, these changes tended to decrease and by 30 days the density of lymph nodes and the size of germinal centers decreased by about a third and amounted to 19.3±0.2% and 20.4±0.2%, respectively.
In the second group, using AB, the most pronounced changes occurred on the day 7 of the experiment. The division of the lymph node into the cortical and medulla layers due to the proliferation of lymphoreticular cells was noted. Hemocytoblasts formed follicles with outlined light centers. The increase in the density of lymphatic nodules per unit area of the sample, as well as the size of germinal centers, was 28.4±0.9% and 31.2±1.1%, respectively (p≤0.05). Fig. 2.
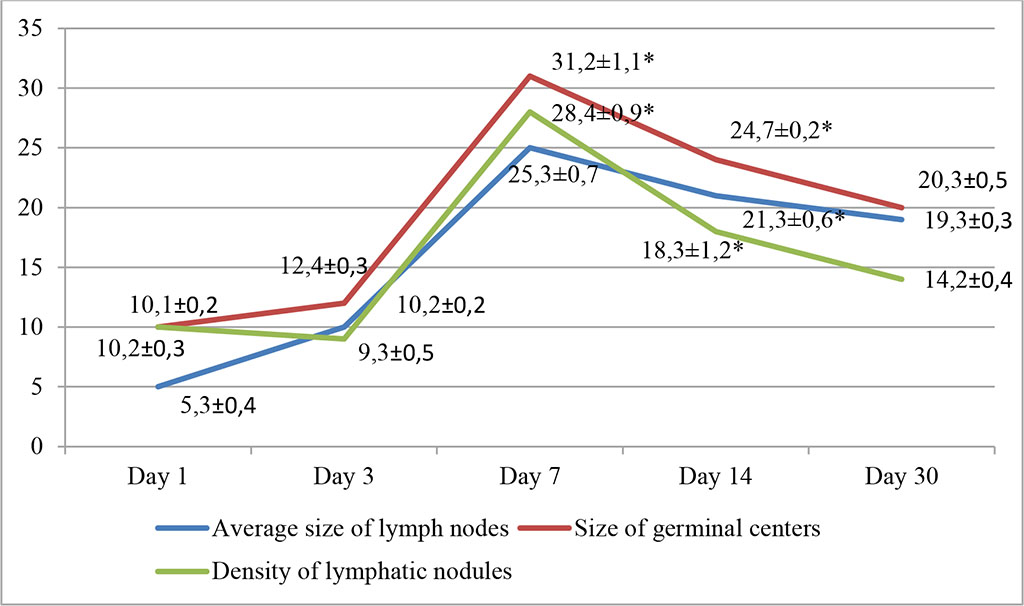
Fig. 2. Dynamics of morphofunctional changes in lymph nodes during intralymphatic administration of AB at different observation dates (%); * - differences are statistically significant at p<0.05
We also noted an increase in the average size of lymph nodes, which was within statistically significant values and amounted to 25.3±0.3%. These changes persisted on the day 14 and amounted to 18.3±1.2%, 24.7±0.2% and 21.3±0.6%, respectively (p≤0.05). An increase in the density of cells in the cortical zone of the lymph nodes was recorded. The relative number of lymphoblasts exceeded the control values by 18.42% (day 7) and 17.82% (day 14). Subsequently, the changes tended to decrease and by 30 days the density of lymphatic nodules and the size of germinal centers decreased.
In the third group, during intralymphatic administration of HP, the most pronounced changes occurred on days 14-30 of the experiment. We noted a rarefaction of the cortical layer with follicular hyperplasia and with formation of clearly defined light centers; proliferation of lymphoreticular cells was noted in the follicles. On the day 14, there was an increase in the density of lymphatic nodules per unit area of the sample, as well as the size of the germinal centers: 35.8±1.7% and 36.5±1.5%, respectively (p≤0.05). There was an increase in the average size of lymph nodes within statistically significant values and amounted to 22.5±0.7%. These changes persisted on the day 14. Fig. 3.
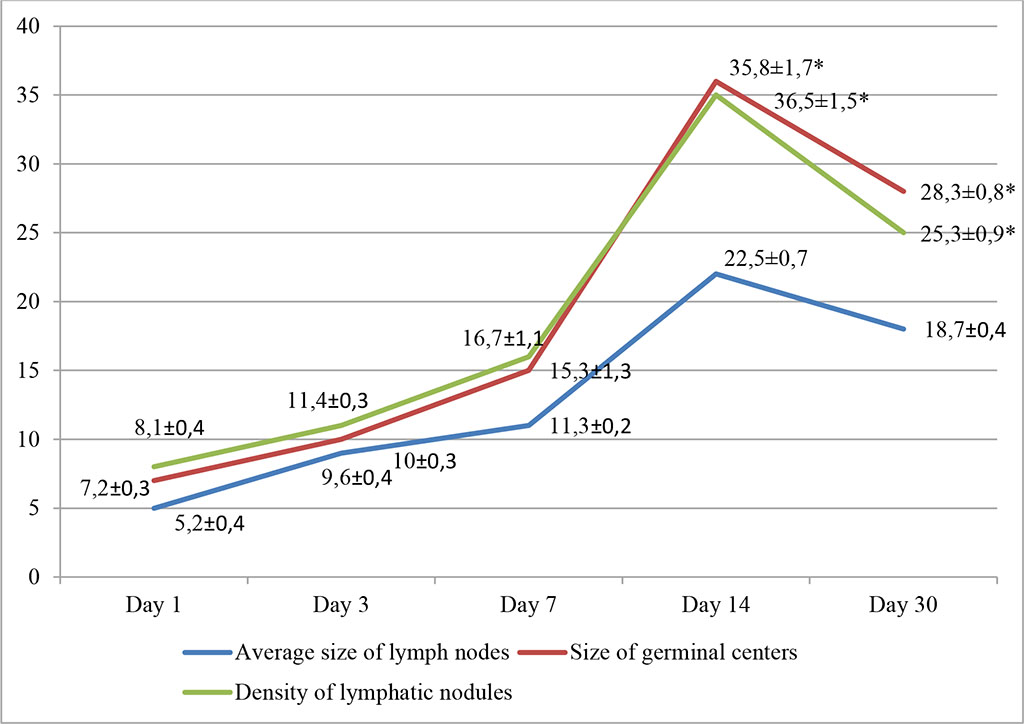
Fig. 3. Dynamics of morphofunctional changes in lymph nodes during intralymphatic administration of HP at different observation dates (%); * - differences are statistically significant at p<0.05
We recorded an increase in the density of cells in the cortical zone of the lymph nodes of experimental animals. The relative number of lymphoblasts exceeded the control values by 17.32% (day 14) and 16.81% (day 30). Subsequently, the changes tended to decrease and by 30 days the density of lymphatic nodules and the size of germinal centers decreased.
In the fourth group, with the introduction of ADPS, changes in the morphofunctional state of the mesenteric lymph nodes of experimental animals were similar to those in the third group. Also, the most pronounced changes occurred on days 14-30 of the experiment. In addition to rarefaction of the cortical layer with follicular hyperplasia and with formation of clearly defined light centers, proliferation of lymphoreticular cells in the follicles was noted. By day 30, there was an increase in the density of lymphatic nodules per unit area of the sample, as well as the size of the germinal centers, amounting to 36.4±0.4% and 31.4±0.7%, respectively (p≤0.05). Fig. 4.
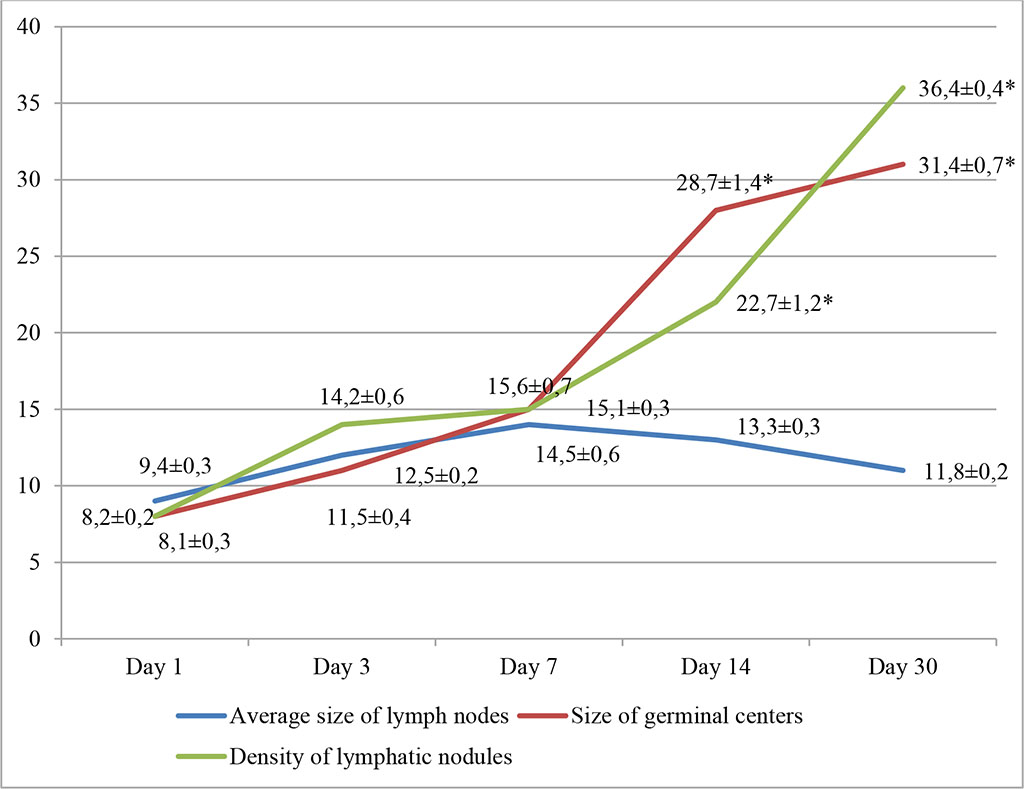
Fig. 4. Dynamics of morphofunctional changes in lymph nodes during lymphotropic administration of ADPS at different observation dates (%); * - differences are statistically significant at p<0.05
The increase in the average size of lymph nodes did not show statistically significant changes. The relative number of lymphoblasts exceeded the control values by 12.36% (day 14) and 14.77% (day 30).
The indicators of the mitotic activity index (Imit) and the migratory activity index (Imig) are presented in Tab. 1 and Tab. 2.
Table 1. Indicators Imit and Imig in groups 1 and 2 at different observation dates
| Indicators | APG | AB | ||||||||
| 1 | 3 | 7 | 14 | 30 | 1 | 3 | 7 | 14 | 30 | |
| Imit | 11,2±0,2 | 13,6±0,2* | 12,6±0,3 | 12,4±0,1 | 12,6±0,2 | 12,1±0,2 | 12,3±0,4 | 13,8*±0,2 | 13,5±0,4 | 12,8±0,1 |
| Imig | 2,8±0,01 | 3,8±0,03* | 3,6±0,04 | 3,1±0,01 | 2,9±0,02 | 2,9±0,05 | 2,8±0,03 | 3,6*±0,06 | 3,4±0,03 | 2,9±0,02 |
(* - differences are statistically significant at p<0.05)
Table 2. Indicators Imit and Imig in groups 3 and 4 at different observation dates
| Indicators | HP | ADPS | ||||||||
| 1 | 3 | 7 | 14 | 30 | 1 | 3 | 7 | 14 | 30 | |
| Imit | 10,2±0,2 | 11,6±0,2 | 11,6±0,3 | 13,4±0,1* | 12,6±0,2* | 11,1±0,3 | 11,3±0,3 | 11,6±0,2 | 12,5±0,4* | 13,8±0,1* |
| Imig | 2,7±0,01 | 2,4±0,03 | 2,8±0,04 | 3,8±0,01* | 3,6±0,02* | 2,2±0,05 | 2,5±0,03 | 2,6±0,06 | 3,4±0,03* | 3,9±0,02* |
(* - differences are statistically significant at p<0.05)
When studying Imit and Imig in experimental groups, it turned out that with the introduction of APG these indicators significantly increased on the day 3 of the experiment. In the group with intralymphatic administration of AB, a statistically significant increase in indicators was noted on the day 7 of the study. In the third and fourth groups, an increase in Imit and Imig occurred only after 14 days of lymphotropic administration.
In the fifth, control group of the study, we didn’t note statistically significant changes in the average size of lymph nodes, the size of germinal centers, the density of lymphatic nodules, Imit and Imig, nor qualitative changes in cellular composition.
When analyzing structural changes in the morphofunctional state of the mesenteric lymph nodes of experimental animals during intralymphatic administration of immunomodulators, we noted changes characterized by morphological restructuring of the lymph nodes in all experimental groups, which was accompanied by an increase in their reactivity and provided an immunostimulating effect. In a dynamic study of the condition of the lymph nodes during the intralymphatic administration of various immunomodulators, it turned out that changes occurred at different dates. With the introduction of APG, the greatest structural changes occurred on the day 3 of the experiment. In the case of intralymphatic injection of AB, peak changes occurred on days 7-14 from the start of administration. In the third and fourth groups, using HP and ADPS, structural restructuring of the lymph nodes occurred on days 14-30.
When extrapolating the data obtained to the clinical plane, we can conclude that intralymphatic administration of APG can be recommended in the acute period of the disease, intralymphatic administration of AB - in the case of subacute course of the disease, and intralymphatic administration of HP and ADPS will be effective during the period of convalescence.