- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 2: e1. DOI 10.35630/2024/14/2.212
Orbital vascular lesions are challenging to treat and observation is often preferred if symptoms are mild. Historically, surgical excision of the lesions used to be the preferred treatment, but intraoperative bleeding and incomplete removal were common. Therefore, sclerotherapy has become the treatment of choice by virtue of being minimally invasive and extremely effective. Sclerotherapy involves multiple intralesional injections of sclerosing agents like bleomycin and sodium tetradecyl sulfate. In this communication, we describe an unusual complication associated with sclerotherapy to treat an orbital venous malformation in a young woman diagnosed with a type-2 low flow venous malformation (orbital varices) in her left orbit. There was complete resolution of the lesion. However, following the procedure, she complained of diplopia accompanied by left hypotropia. The condition persisted six months after the procedure. Forced duction test confirmed a tight inferior rectus and she refused any further intervention. This case report discusses the possible mechanisms of this rare and unusual complication associated with sclerotherapy while treating orbital vascular lesions.
Keywords: Orbit, Vascular Lesion, sclerotherapy, venous malformation
Orbital vascular malformations (OVM) are a rare but challenging group of vascular anomalies that can lead to significant morbidity if not appropriately managed. OVM are typically classified depending on their hemodynamic flow. Low-flow OVM are characterized by slow-flowing venous channels lined by thin-walled endothelial cells, whereas high-flow OVM are characterized by arteriovenous shunting and thick-walled vessels.[1,2]
Low flow OVM can usually be treated with sclerotherapy, a minimally invasive technique that involves the injection of a sclerosant agent into the venous malformation. The sclerosant causes fibrosis and eventual occlusion. Historically, many liquid based sclerosants such as ethanol, bleomycin, sodium tetradecyl sulfate (STS) and pingyangmycin among others have been effectively used for treating orbital vascular malformations.[3,4] Sclerotherapy is a safe and effective treatment option for low flow OVM, with high success rates and low complication rates.[1,5]
A 38-year-old woman presented to the Oculoplastics Unit with a 2-year history of left eye fullness and pressure while bending (Figure 1).
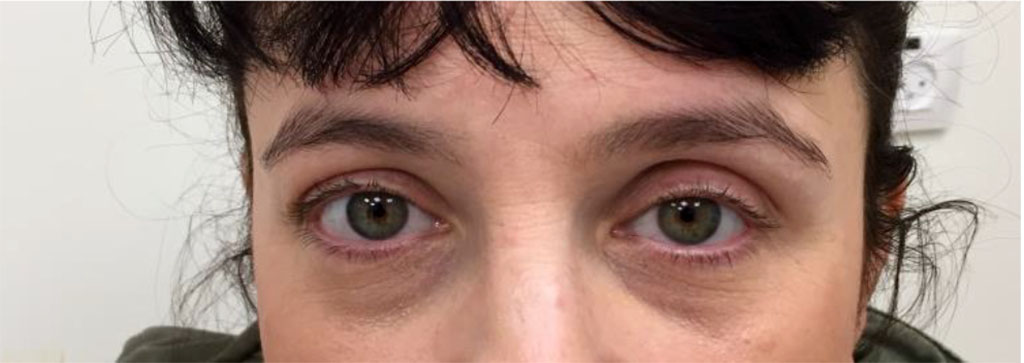
Figure 1: Clinical photo on presentation demonstrating hypoglobus and superior sulcus defect on the left side
Her symptoms began and increased during her second pregnancy. She was not pregnant at the time of presentation. Magnetic resonance imaging (MRI) with Gadolinium and fat suppression revealed a large vascular lesion in the left orbit (Figures 2a,b).
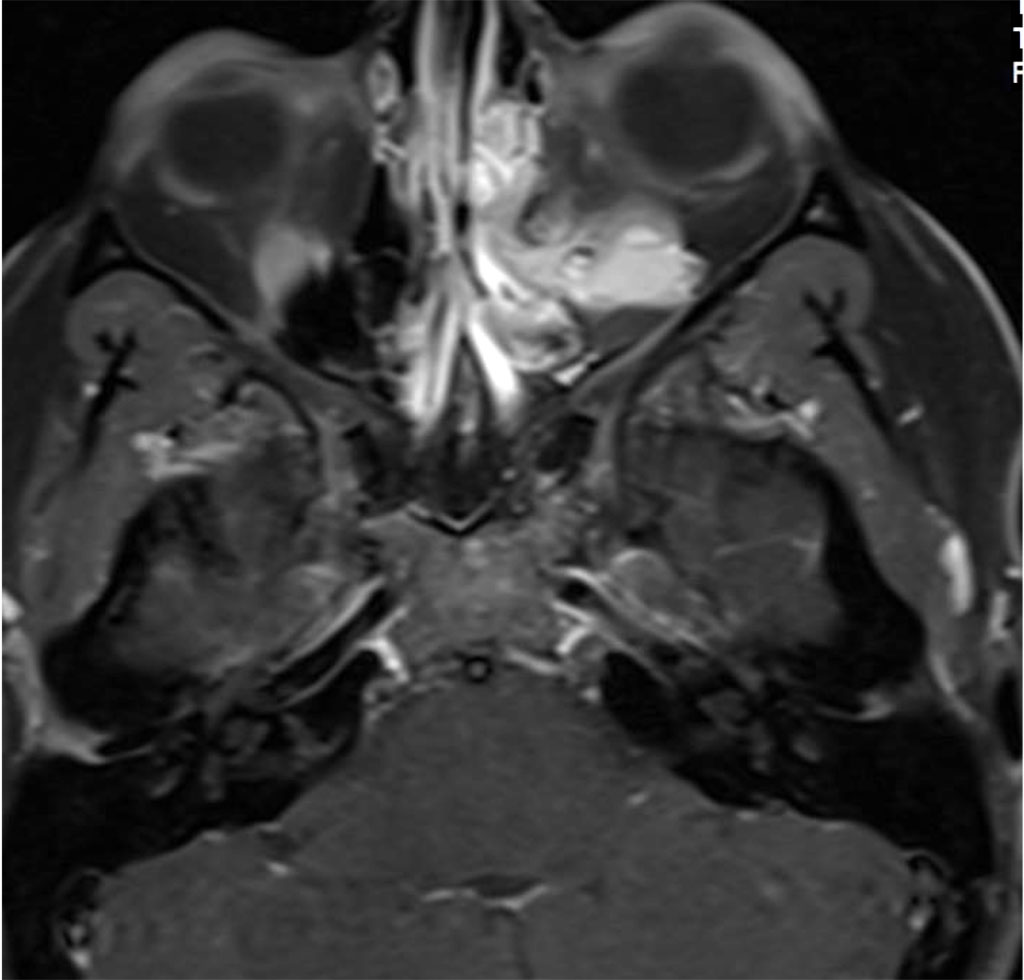
Figure 2a: Axial MR T1 with contrast demonstrating intra and extraconal orbital lesion

Figure 2b: Sagital MR T1 demonstrating left inferior orbital lesion
An endoscopic biopsy was performed and pathology revealed a low flow venous malformation. The patient was about to begin a round of fertility treatments soon. When discussed with her treating physician, there was a suspicion that excessive hormones and pregnancy could potentially cause an increase in the size of this lesion. Additionally, if needed, surgical intervention may not be possible due to the risks associated with advanced pregnancy. After some deliberation, it was decided to treat the lesion with sclerotherapy. Sodium tetradecyl was injected percutaneously under fluoroscopic guidance into the lesion in an uneventful procedure. (Figure 3)
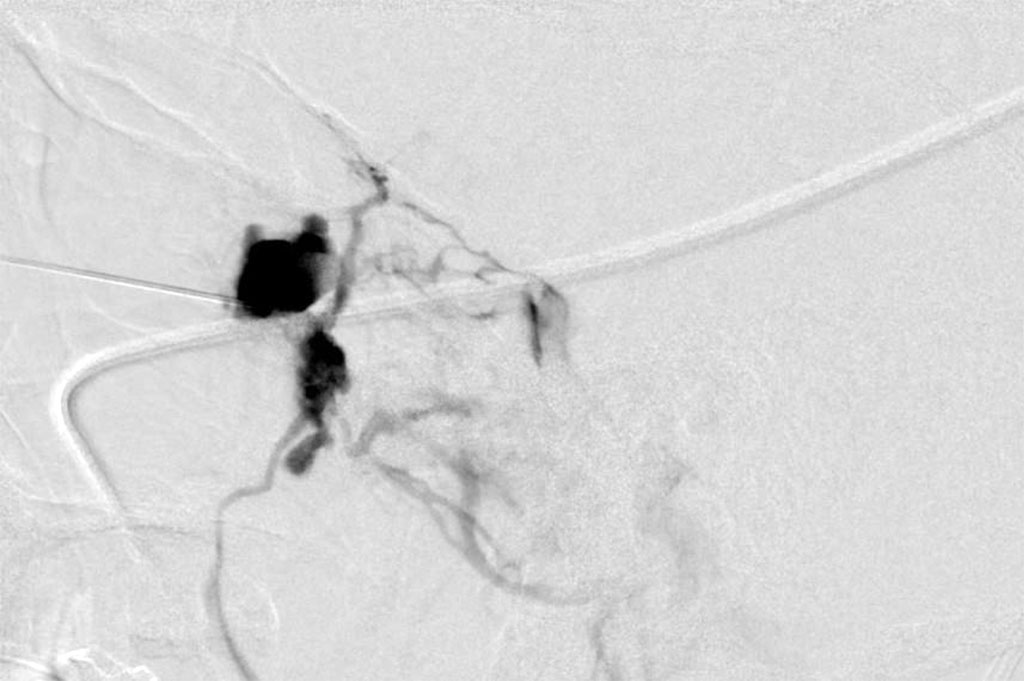
Figure 3: Sclerotherapy – showing sclerosing agent in the vessels of the lesion
Following the procedure, the patient complained of diplopia in primary position with restriction of upgaze. The patient was treated with oral steroids with no improvement. MRI was 2 months post procedure, there was excellent anatomical improvement, with significant decrease of the lesion but with enlargement of the inferior rectus (Figures 4 a,b,c).
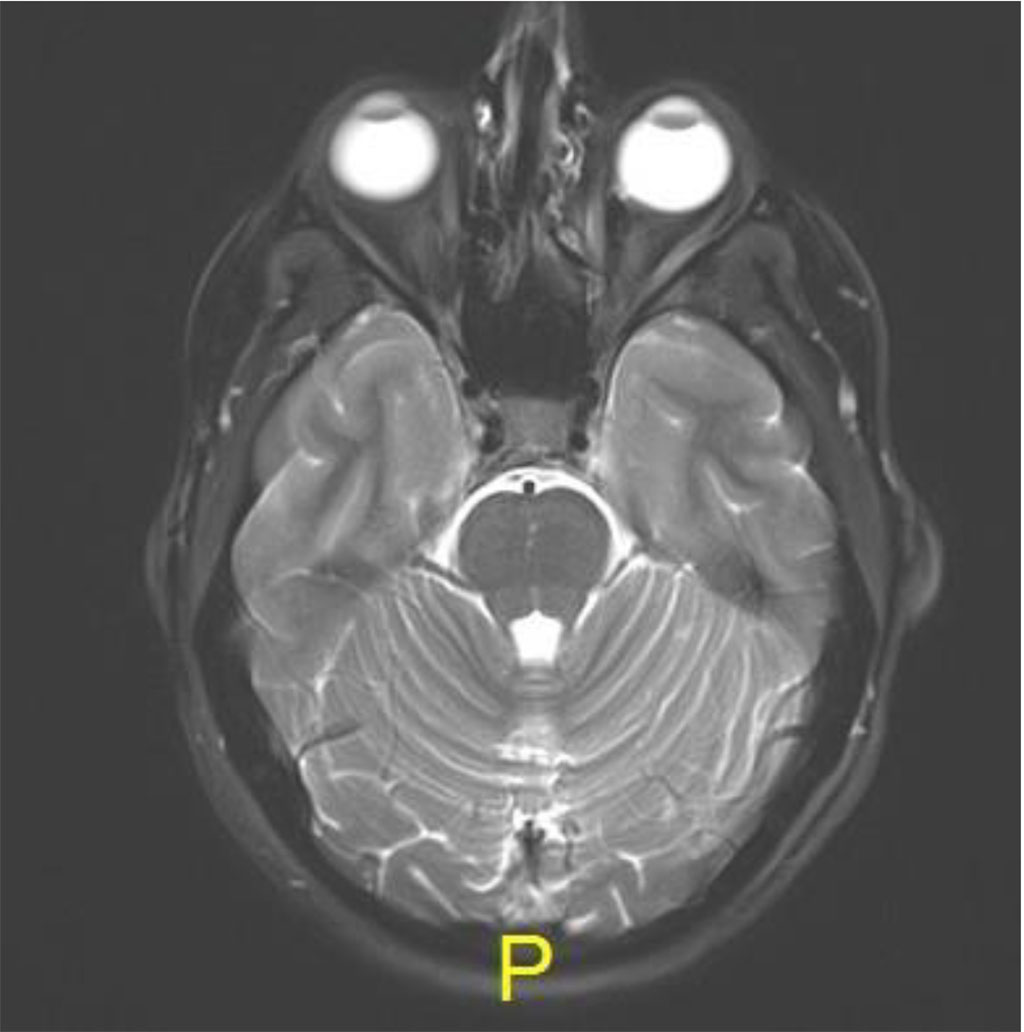
Figure 4a: Axial MR T2: resolution of lesion
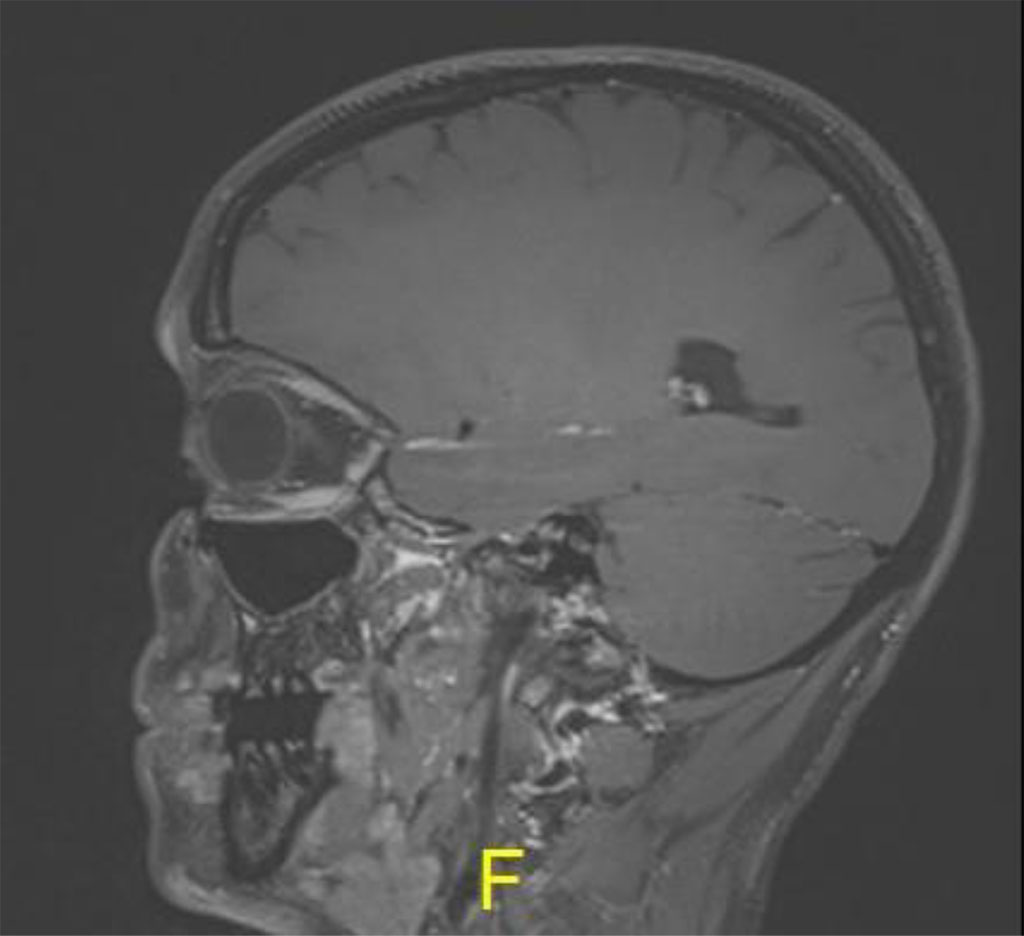
Figure 4b: Sagital MR T1: demonstrating thickening of inferior rectus in left
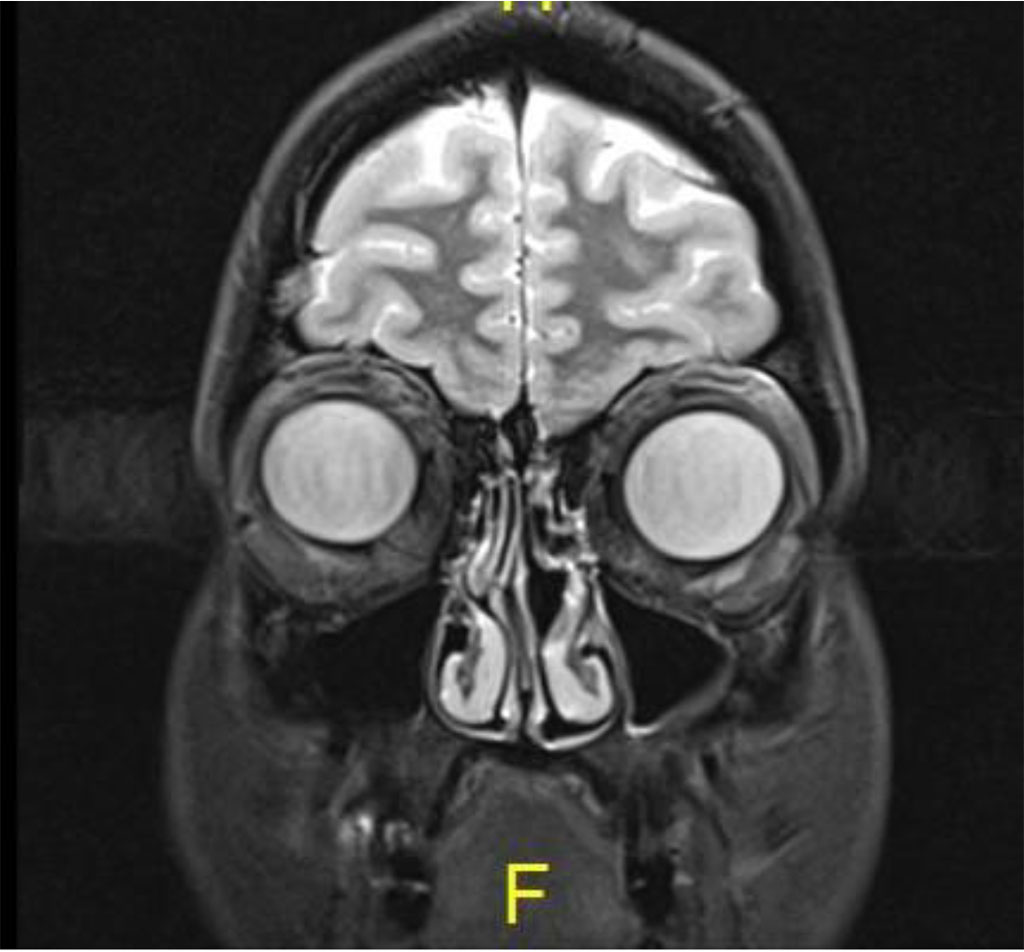
Figure 4c: Coronal MR T2: Complete resolution of lesion
At her last follow up 6 months post sclerotherapy there was still significant primary position diplopia and the patient refused further suggested treatment including prisms and strabismus consult (Figure 5).
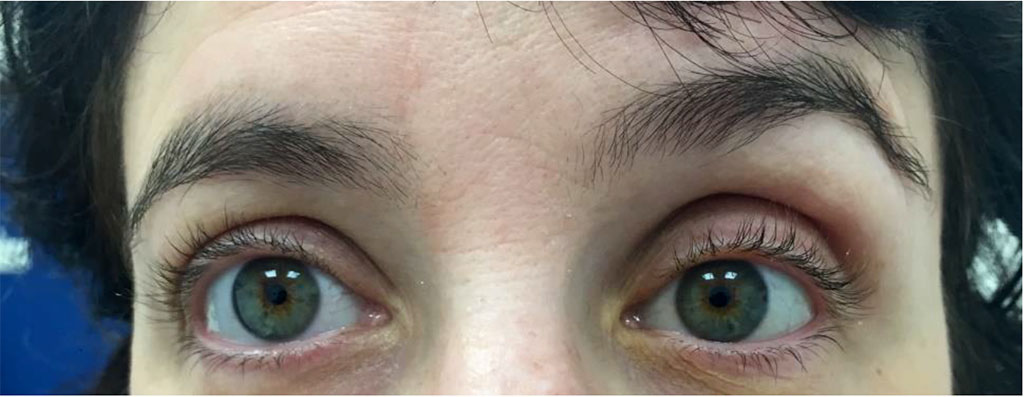
Figure 5: Clinical photo post sclerotherapy demonstrating left hypoglobus
STS has been shown to be very effective as a sclerosant agent. STS is a synthetic surfactant with US Food and Drug Administration (FDA) approval as a sclerosing agent.[6] Intravenous injection of STS incites intimal inflammation and thrombus formation. This leads to obliteration and occlusion of the lumen. The resultant fibrous tissue causes venous obliteration. STS has been used in the treatment of orbital varices and other low flow vascular malformations as well. [7] In our case, even though the fluoroscopic guidance was used during the procedure to secure the position of the catheter through which the sclerosant was injected, we postulate that some amount of the sclerosant may have extravasated outside of the lumen of the vessels and come in contact with the surrounding tissue in the vicinity of the inferior rectus.
When used in the treatment of superficial vascular malformations, it has been reported that extravasation of the sclerosant leads to skin necrosis.[8] While it is possible that introduction of the needle itself could have damaged the inferior rectus muscle, we believe that an inadvertent extravasation of STS from within the injection VLM – outside in the orbit could have incited an inflammatory reaction leading to localized focal necrosis. The resultant myositis and subsequent resolution of inflammation may have led to scarring and resultant shortening of the inferior rectus muscle. The forced duction test performed subsequently, confirmed the presence of a tight inferior rectus muscle helped us out a neurological component and it possibly validates the above explained scenario. Oral corticosteroids were given as a part of the post-operative treatment regimen and this could have helped in limiting the damage.
Extraocular muscle damage and fibrosis or damage has not been described as a complication of using sclerosants for OVM. While fluoroscopic visualization can help in precise injection of the sclerosant; local extravasation remains a distinct possibility and clinicians should be aware of rare and unusual complication of EOM fibrosis arising because of STS use within the orbit.
The authors report there are no competing interests to declare.
There was no funding received for this research and writing of this manuscript.