- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 4: e1. DOI 10.35630/2199-885X/2022/12/4.16
Lichen planus refers to chronic dermatoses with manifestation in the oral mucosa. Its clinical manifestations are displayed as disruption of the immune system balance. A prolonged course of lichen planus causes deregulation of the cell growth gene, dysplasia of the epithelium of the oral mucosa and the development of squamous cell carcinoma. Identification of circulating biological markers in blood and saliva enables to detect stable and reliable predictors of clinical significance in the diagnosis and the prognosis of diseases of the oral mucosa, including the onset of tumor transformation. Squamous cell carcinoma (SCC) antigen is one of these cancer markers. Saliva is an "ultrafiltrate of blood plasma", which makes it possible to evaluate biological markers by a safe non-invasive method. The aim of this study is to assess the concentration of squamous cell carcinoma antigen in mixed saliva samples of patients with various forms of lichen planus at the stages of diagnosis and treatment. Materials and methods. 47 women with a clinical diagnosis of "oral lichen planus" were examined and randomized in groups. They described the dental status, identified the causes of chronic injury, and made a comprehensive treatment plan. Monitoring was carried out at the diagnostic stage, after 14 and 90 days. During the first visit and after treatment, unstimulated mixed saliva was collected on an empty stomach. Results. During the diagnosis, the content of SC in the oral fluid of patients with lichen planus was <500 mcg/ml (489 mcg/ml) and a decrease in its concentration after treatment is characteristic of a benign course and susceptibility to therapy. The concentration of the marker >500 mcg/ml (527 mcg/ml) and an increase in the value after the treatment course indicates a severe recurrent nature of the disease and low efficacy of prescribed medications. Conclusion. The study of squamous cell carcinoma (SCC) antigen in oral fluid expands the applications of the biomarker in determining the prognosis of the course and effectiveness of therapy for oral lichen planus.
Keywords: oral lichen planus (OLP), biomarkers of oral fluid (mixed saliva), squamous cell carcinoma (SCC) antigen.
Lichen planus refers to chronic dermatoses and has manifestations on the mucous membranes of the mouth in the form of papules with the addition of an inflammatory process of varying severity, accounts for 35% of diseases of the mucous membranes of the oral cavity [1, 2]. Currently, in the etiopathogenesis of lichen planus, the leading factor is seen as the immunological reaction of delayed hypersensitivity, which affects the manifestation and is closely related to the occurrence of relapses of the disease [2]. The imbalance of the immune system underlies the clinical manifestations of lichen planus [4-7]. At the same time, many researchers suggest that the mechanisms of lichen planus development are associated with a decrease in immunosuppression against the background of somatic pathology, and as a consequence, a pronounced hyperergic inflammatory reaction [1-6].
Yu.N. Perlamutrov et al. the cytokine profile of mixed saliva of patients with exudative hyperemic and erosive ulcerative forms of lichen planus was studied, an increase in the content of interleukin 4 (IL-4) was established by 2 times and proved the effectiveness of systemic immunosuppressive therapy, against which the level of this indicator returned to the values of the control group [7].
Yu.M. Fedotova et al. in patients with erosive and ulcerative form of lichen planus, an increase in the concentration of IL-4 in the oral fluid was detected at all stages of follow-up (from 3 to 365 days) after therapy with the inclusion of a bioadhesive drug with anti-inflammatory effect [8].
According to S.G. Fitzpatrick, a prolonged course of lichen planus leads to a violation of the regulation of cell growth, the appearance of apoptic keratinocytes, which manifests itself in the proliferation of keratoplastic deposits, dysplasia of the epithelium of the oral mucosa and the subsequent development of squamous cell carcinoma [9]. It should be noted that neoplastic transformation as a possible outcome of lichen planus justified the inclusion of this disease in the classification of precancerous conditions of the oral mucosa [5, 9, 10].
Identification of circulating biological markers in blood and saliva makes it possible to detect predictors of clinical significance in the diagnosis and prognosis of diseases of the oral mucosa, including the initial stages of tumor development [11, 12]. Squamous cell carcinoma antigen (SCC), which is a glycoprotein, a subtype of the TA-4 antigen associated with a tumor, synthesized by epithelial cells of the mucous membrane, is considered one of such cancer markers [13-17]. Two isoforms are isolated in blood plasma: SC1 and SC2. At the same time, IL-4 increases the synthesis of type 1 SCC-Ag, and IL-13 activates the production of type 2 SCC-Ag [14-18].
A dynamic study of SCC-Ag concentration is used to diagnose cancer of the oral mucosa, cervix, esophagus, lungs with a specificity of more than 80% and to monitor the response to treatment [19], as well as to assess the prognosis of patient survival [20, 21].
Some researchers analyzed the role of single- and multifactorial statistical methods in analyzing the relationship between an increase in SCC-Ag and the risk of relapse, as well as the timely detection of metastases during treatment and established a significant correlation [20-25].
Multivariate analysis showed that relapse and low survival of patients with cancer of the oral mucosa and larynx is characteristic when the concentration of SCC-Ag ≥4 ng/ml is exceeded [22-24].
At the same time, high levels of SCC-Ag (≥2.0 ng/ml) and C-reactive protein (≥5.0 mg/L) serve as a statistically significant marker of the tumor, the presence of distant metastases and determine the patient's survival at the stage of diagnosis of relapse [23]. The overall increase in the levels of SCC-Ag and C-reactive protein is associated with the prevalence of the process and metastatic tumor factors [19-25].
Saliva is considered an "ultrafiltrate of blood plasma", which makes it possible to evaluate biological markers by a safe non-invasive method [26, 27]. Oral fluid washes the elements of the affected mucous membranes of the oral cavity and contains certain levels of biological markers, which can be used in disease monitoring [26-30].
There is no information available in the literature on the study of the concentration of SCC in mixed saliva in patients with precancerous conditions of the oral mucosa, which determined the scope of this study.
The aim of this study is to assess the concentration of squamous cell carcinoma antigen in mixed saliva samples of patients with various forms of lichen planus at the stages of diagnosis and treatment.
In 2019-2021, 47 women (35-70 years old) with a clinical diagnosis of oral lichen planus, ICD – "L 43 Lichen red flat" were examined at the Department of Dentistry of Central State Medical Academy (Moscow, Russia) . The patients were randomized into groups depending on the form of lichen planus (with a low and potential possibility of tumor transformation) and the treatment protocol:
The persons with diseases of the oral mucosa: infectious, allergic, benign tumors, leukoplakia were excluded from the study. The control group included 19 patients aged 45-65 years without lesions of the oral mucosa.
Standard methods were used to collect complaints and anamnesis of the disease, during external examination, attention was focused on the condition of the skin, the red border of the lips, and the lymph nodes of the regional region. In the oral cavity, the status of the mucous membrane of the lips, cheeks, hard and soft palate, gums, and teeth were described. The causes of chronic trauma (sharp edges of destroyed and dystopian teeth, fillings, orthopedic structures) were identified.
All patients were recommended to avoid sharp, sour, spicy foods and observe the temperature regime of food intake, carried out professional hygiene, eliminated traumatic factors, replaced substandard fillings, orthopedic structures.
Taking into account somatic pathology, a comprehensive individual treatment plan was prepared for each subject with the appointments of doctors of related specialties (dermatologist, neurologist, therapist, gastroenterologist, cardiologist), focused on the pathogenetic links of lichen planus. The course of therapy necessarily included the appointment of antioxidants, sedatives, vitamins.
Local treatment of typical and exudative hyperemic forms of lichen planus consisted in the use of oral baths of an oil solution of natural origin based on a complex of biologically active substances from pumpkin seeds with anti-inflammatory, regenerating, metabolic, antioxidant properties for 1 tablespoon, 1 minute, 2 times a day after meals. Patients with erosive and ulcerative form of lichen planus were added mouthwash with antiseptic solution 10-15 ml, 3-4 times a day, application of calcineurin inhibitor ointment, synthesis and release of proinflammatory cytokines on morphological elements of the oral mucosa for 10-15 minutes, 2 times a day [7, 33]. The duration of the course of therapy was 2 weeks.
Monitoring was carried out at the diagnostic stage, after 14 and 90 days.
At the first visit and after treatment on an empty stomach, unstimulated mixed saliva was collected in a graduated 5 ml tube, then frozen and transported to the Laboratory of Clinical Biochemistry of the N.N. Blokhin National Research Institute of Oncology of the Ministry of Health of Russia. The concentration of SCC in the oral fluid was determined by enzyme immunoassay using kits "CanAg SCC EIA" ("Fujirebio Diagnostics AB", Sweden) in accordance with the manufacturer's instructions. Due to the high concentration of SC in saliva detected in test studies, the samples were pre-diluted 100-fold with blood serum with a known low SCC content, which was taken into account in subsequent calculations of marker levels. The marker content was expressed in micrograms per 1 ml (mcg/ml).
Statistical processing of the results was carried out using the central characteristic – median, quartiles were used to estimate the spread of indicators, nonparametric analysis methods were used for comparison: Mann-Whitney (U test) and the Wilcoxon paired test. The differences were considered statistically significant at p <0.05.
In group 1, at the diagnostic stage, the transitional fold, the line of teeth closure in the distal parts of the mouth, the retromolar region on the unchanged mucous membrane of the oral cavity were marked with grayish-white papules, rough, protruding above the color–unchanged mucous membrane in the typical form; on the hyperemic and edematous mucous membrane - in group 2 (Fig. 1, 2 (a, b).
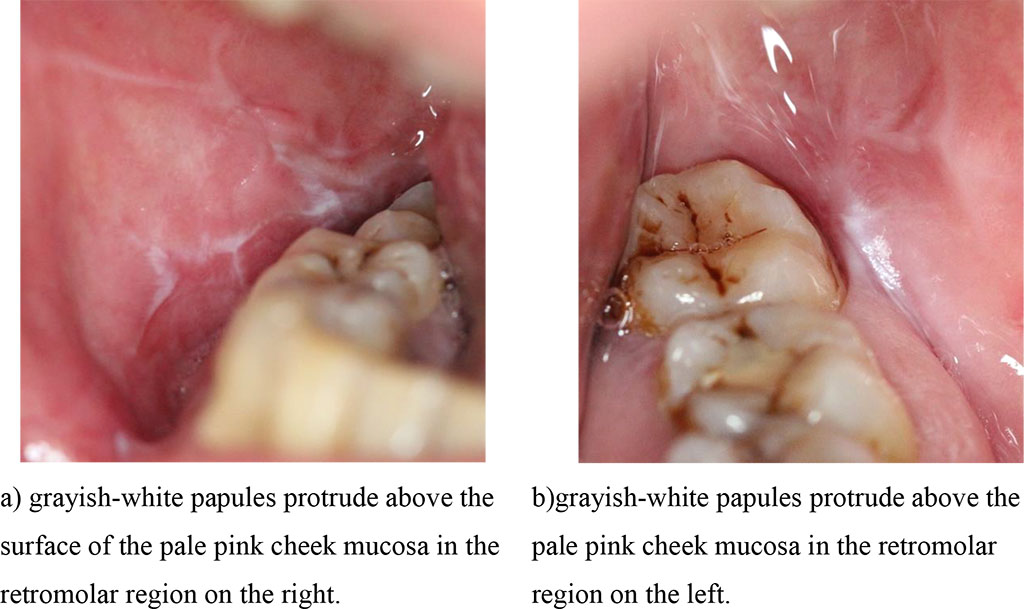
Fig.1 (a, b). Clinical manifestations of a typical form of lichen planus before treatment.
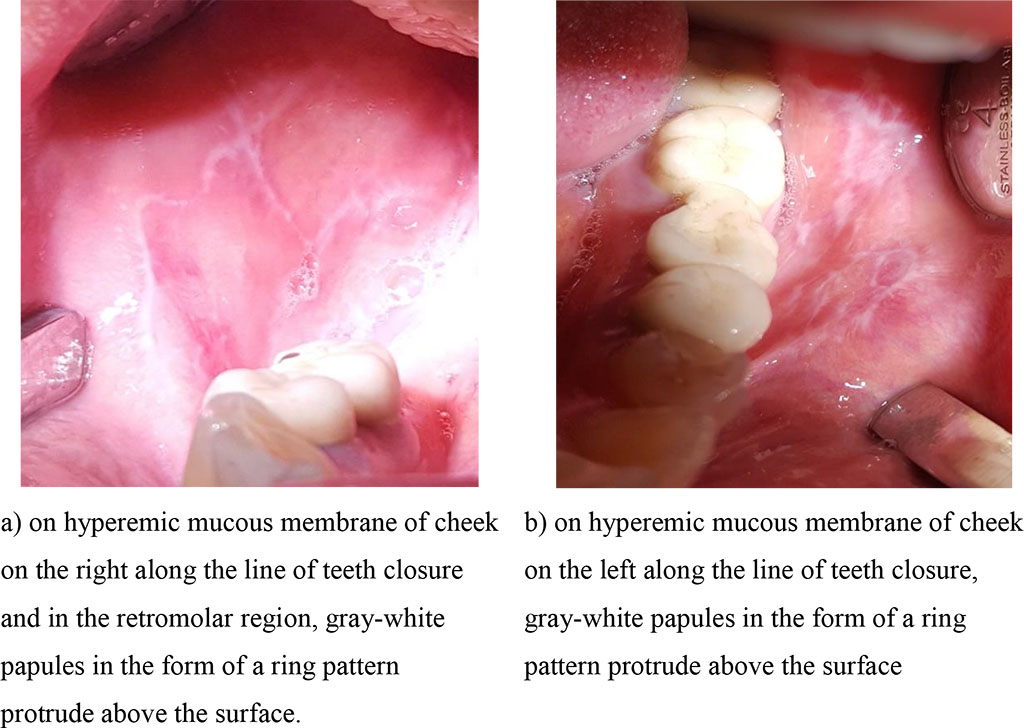
Fig. 2 (a, b). Clinical manifestations of the exudative hyperemic form of lichen planus before treatment
In group 3 (erosive-ulcerative form of lichen planus), during examination in the distal parts of the mouth, merging erosions, irregular shapes, covered with fibrinous plaque, painful, soft-elastic consistency and grayish-white papules, rough, towering over the hyperemic surface of the mucous membrane were diagnosed (Fig. 3 (a, b).
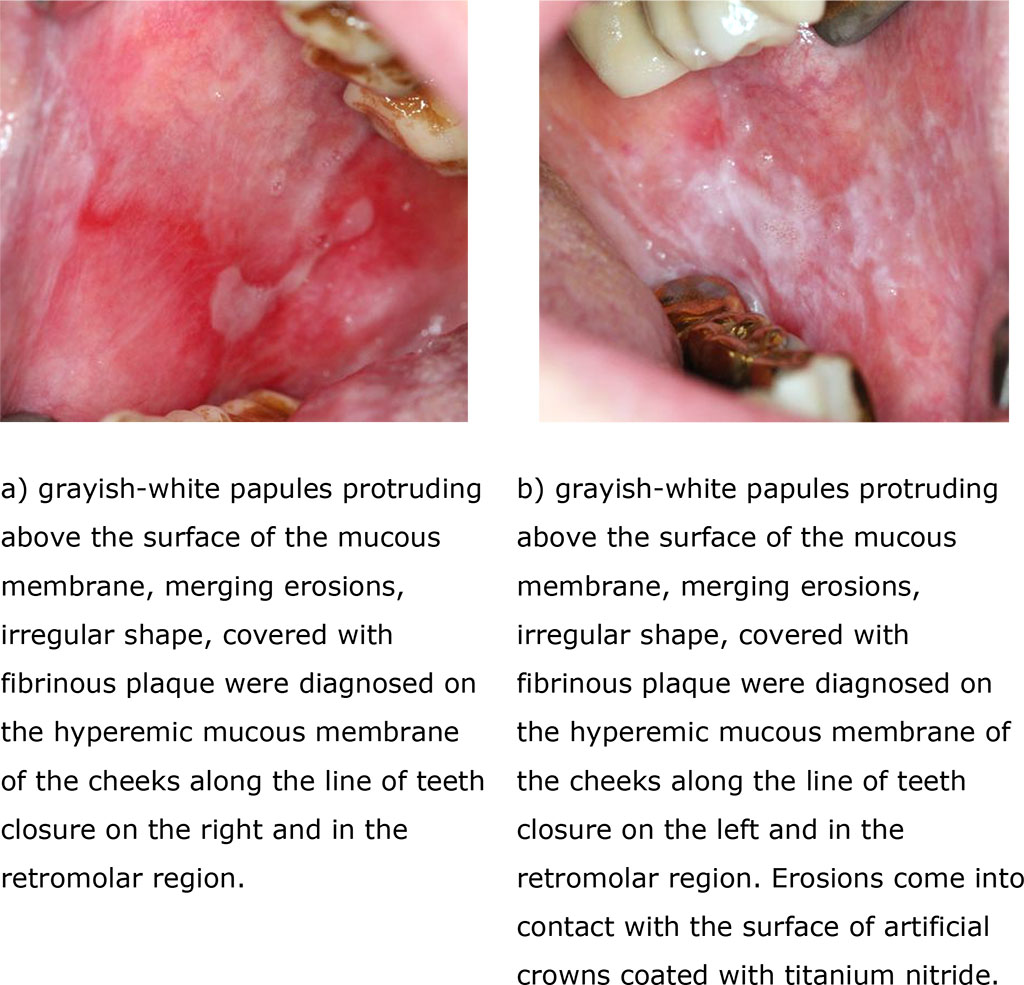
Fig. 3 (a, b). Clinical manifestations of erosive-ulcerative form of lichen planus before treatment
At the first visit, in group 1 patients with typical lichen planus, an excess of the median concentration of oral fluid SCC was determined, the value was 468 mcg/ml (299-505 mcg/ml) and statistically significantly twice the corresponding control indicator (232 mcg/ml; p=0.055).
In patients with exudative hyperemic lichen planus (group 2), the median SCC level in mixed saliva before treatment was 489 mcg/ml and exceeded the control level by more than 2 times. At the same time, SCC levels in the typical and exudative-hyperemic form of lichen planus practically did not differ.
A statistically significant increase in the concentration of SCC in saliva before treatment compared with the control was also noted in patients with erosive-ulcerative form of ringworm (p=0.01): medians 527 and 232 micrograms/ml, respectively (Table 1).
Table 1.The content of SC in the oral fluid of patients with various forms of lichen planus and in control.
| Groups | N | SCC, mcg/ml | ||
| Limits | Median | Quartiles | ||
| Control | 19 | 97,8-985 | 2321 | 119-479 |
| Group 1 (typical) | 10 | 36-784 | 4681 | 299-505 |
| Group 2 (exudative hyperemic) | 14 | 36,5-917 | 4891 | 293-784 |
| Group 3 (erosive and ulcerative) | 23 | 219-2264 | 5272 | 260-1340 |
Note1: – p = 0.05 compared to the control (U test); 2 – p = 0.01 compared to the control (U test);
Analysis of the effect of therapeutic measures on the concentration of SCC in the oral fluid in groups 1 and 2 showed the following pattern: a 100% decrease in the values of the marker relative to its initial levels in patients with typical manifestations of lichen planus and in 67% - with exudative hyperemic form. Clinical examination after 90 days revealed no recurrence of the disease in these patients with lichen planus (Pic. 4, 5 (a, b).
However, 33% of group 2 patients after treatment showed an increase in SCC, the indicator was within the upper limits of 738 mcg/ml, during the control examination, they had edema and hyperemia of the mucous membrane.
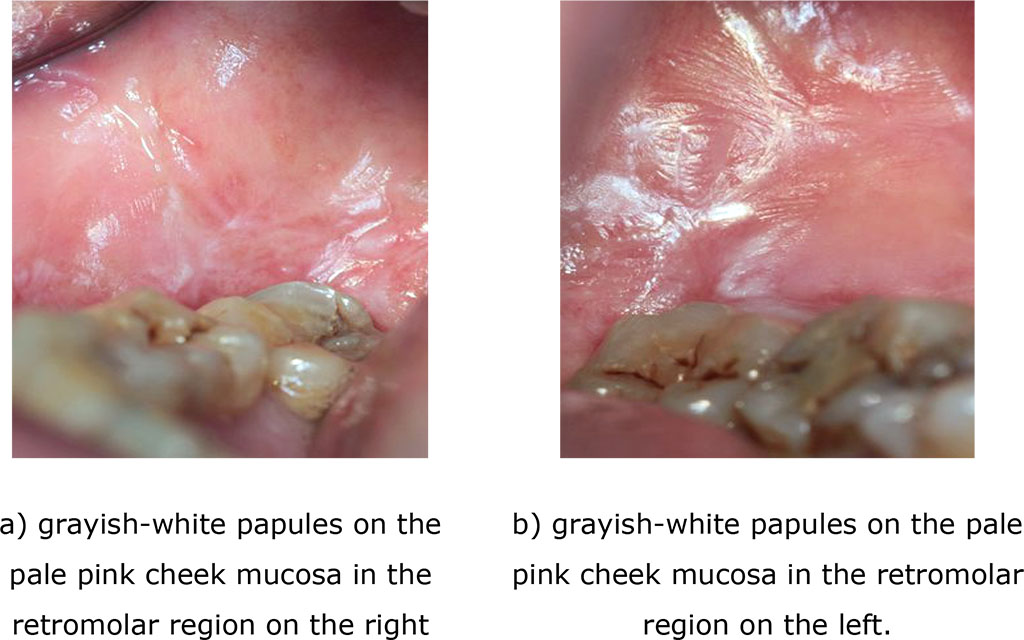
Fig. 4 (a, b). Clinical manifestations of a typical form of lichen planus after treatment (after 14 days)
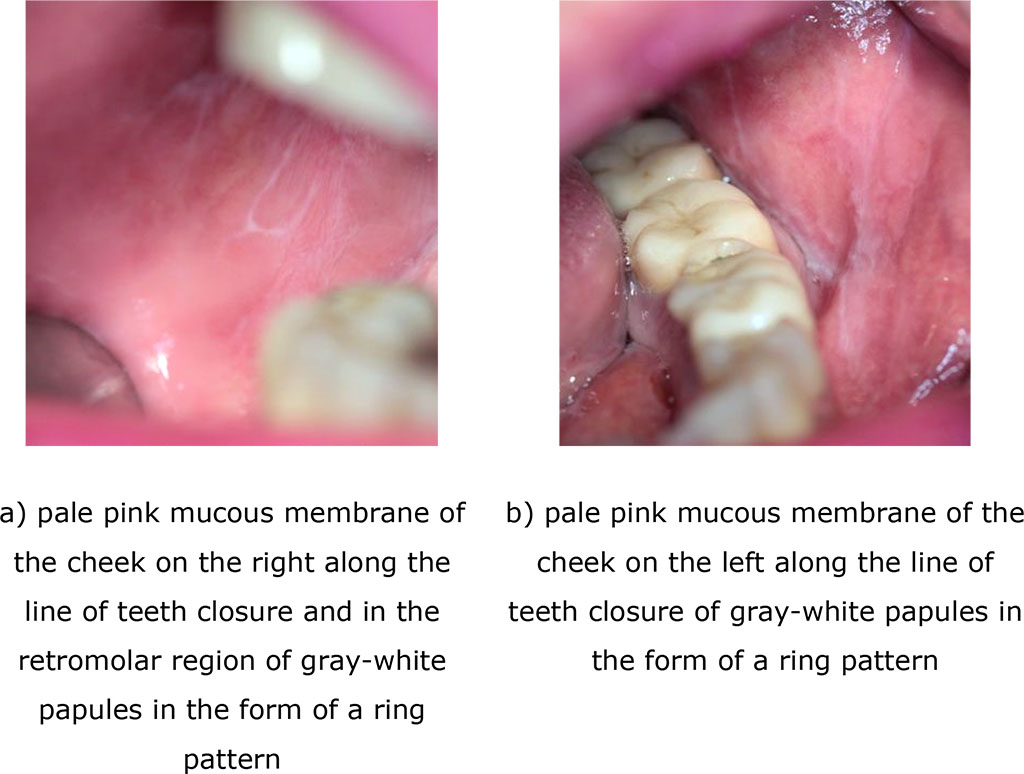
Fig. 5 (a, b). Clinical manifestations of the exudative hyperemic form of lichen planus after treatment (14 days)
In group 3, after treatment, 80% of patients showed a marked increase in the concentration of SCC in mixed saliva compared to the baseline. The median concentration of the marker increased to 811 micrograms/ml, while the upper limit of values reached 2697 micrograms/ml (p=0.08). After 90 days, these patients had an exacerbation of lichen planus with the appearance of complaints of burning and pain when eating (Pic. 7 a, b), as well as erosions on the hyperemic, edematous mucous membrane. At the same time, in the remaining 20% of patients, a decrease in the level of SCC to 269 mcg/ml was recorded and no exacerbation of the disease was noted during the entire follow-up period.
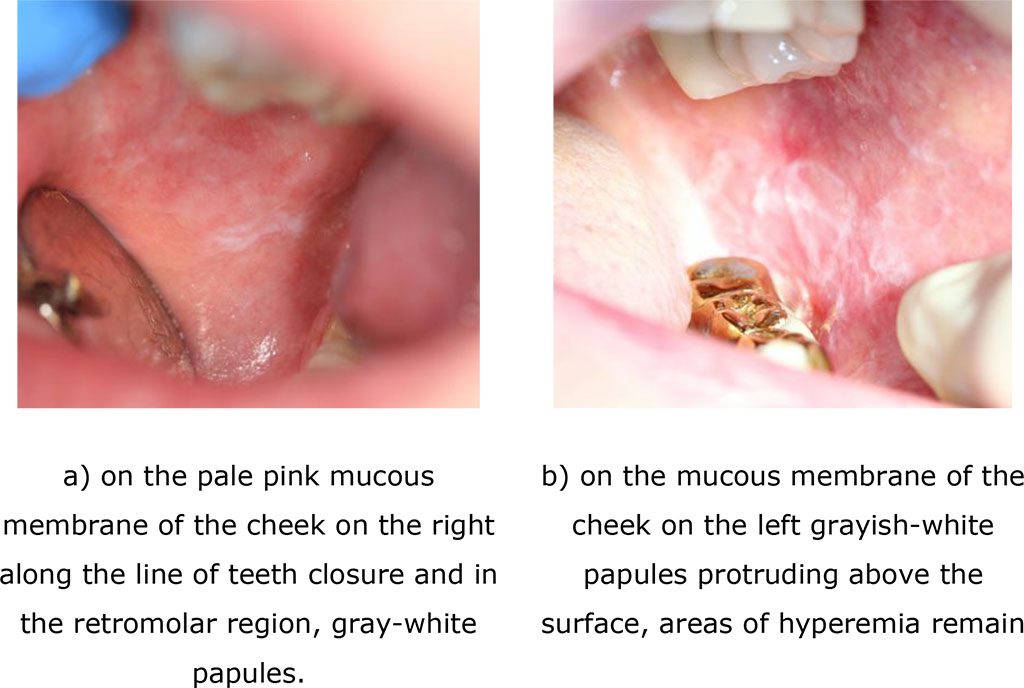
Fig. 6. Clinical manifestations of erosive-ulcerative form of lichen planus after treatment (after 14 days)
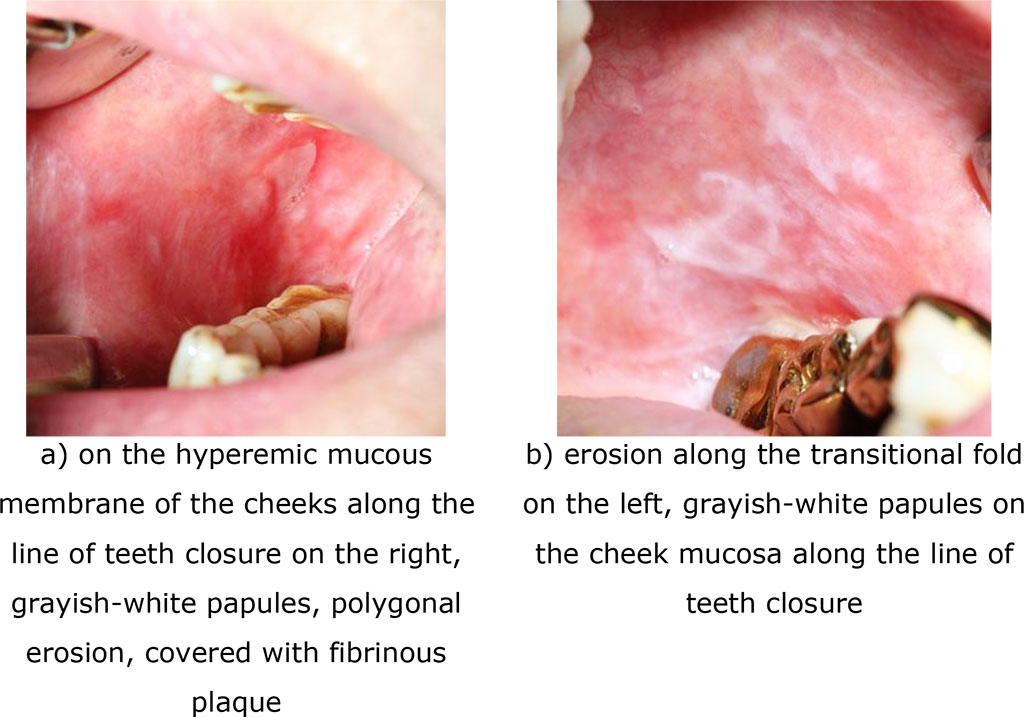
Fig.7. Clinical manifestations of erosive-ulcerative form of lichen planus (exacerbation) after 90 days
Таble. 2.The content of SCC in the oral fluid of patients with various forms of lichen planus after treatment and in control
| Groups | N | SCC, mcg/ml | ||
| Limits | Median | Quartiles | ||
| Group 1 (typical) | 10 | 32-698 | 3652,3 | 191-569 |
| Group 2 (exudative hyperemic) | 14 | 323-738 | 4602,3 | 345-690 |
| Group 3 (erosive and ulcerative) | 23 | 269-2697 | 8112,3 | 360-908 |
Note1: – p = 0.01 compared to the control (U test); 2 – p = 0.08 compared to the indicator before treatment (Wilcoxon test)
So, at the stage of diagnosis, the content of SCC in the oral fluid of patients with lichen planus <500 mcg/ml (489 mcg/ml) and a decrease in its concentration after treatment is characteristic of a benign course and susceptibility to therapy.
The concentration of the marker >500 mcg/ml (527 mcg/ml) and an increase in the value after the treatment course indicates a severe recurrent nature of the disease and low efficacy of prescribed medications (patent application 2022114064).
The excess of SCC levels in mixed saliva relative to the initial ones after treatment in 33% of patients with exudative hyperemic and 80% with erosive ulcerative forms of lichen planus (marker values were in the upper limits of values) indicated pronounced clinical manifestations of the disease after 90 days.
The results obtained can be explained as follows. The content of SCC has a direct relationship with the levels of certain interleukins (IL-4, IL-13) in the oral fluid.[18]. Activation of SCC-1 occurs due to an increase in IL-4, which in turn interacts with mast cells that secrete tumor necrosis factor TNF-alpha, which causes the destruction of the basal cell membranes and the development of an inflammatory reaction. The second form of SCC is induced by IL-13, which has similar biological activity to IL-4 and is produced by differentiated cytotoxic T-lymphocytes, activated B-lymphocytes and mast cells. The listed immunocompetent cells are present in the active phase of lichen planus disease [1,2,4,5,7,8]. In addition, high levels of SCC may indicate the severity of an allergic and autoimmune component in the etiopathogenesis of lichen planus [27]. Thus, the dynamics of SCC levels in the oral fluid is closely related to the severity of inflammatory phenomena of the oral mucosa.
A decrease in the concentration of SCC in mixed saliva after treatment relative to the initial values of the marker confirms a long period of remission, and its increase, on the contrary, indicates a relapse of the disease after 90 days.
The revealed trend justifies the need for systemic use of immunosuppressive drugs (intramuscularly or orally) as pathogenetic therapy aimed at blocking cytotoxic reactions [2,7, 31]. The inclusion of only local immunotropic agents in the treatment regimen partially relieves the inflammatory process of the oral mucosa [4,7,10].
So, the study of SCC in oral fluid expands the scope of the biomarker in determining the prognosis of the course and effectiveness of therapy for lichen planus of the oral mucosa.